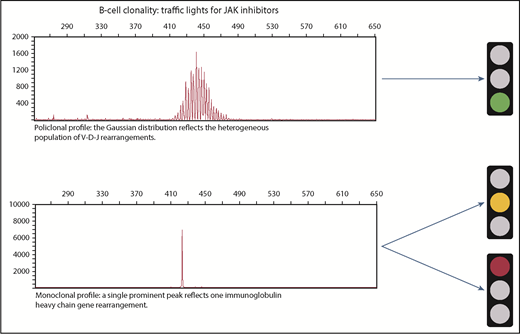
In this issue of Blood, Pemmaraju et al explored the potential correlation between therapy with JAK inhibitors and the risk of a subsequent lymphoma in patients with myeloproliferative neoplasms (MPNs).1
Immunoglobulin rearrangements and therapeutic decisions regarding treatment with JAK inhibitors.
Immunoglobulin rearrangements and therapeutic decisions regarding treatment with JAK inhibitors.
MPNs are clonal disorders complicated by vascular events, myelofibrosis, transformation to leukemia and, more rarely, the development of secondary malignancies.2 Lymphoproliferative neoplasms (LPNs) in patients with MPNs are rare but occur at a higher frequency than that found in the general population.3-7 A study published in August 2018 raised the possibility that treatment for myelofibrosis using JAK inhibitors was associated with an increased risk for aggressive B-cell lymphomas, suggesting that detection of a preexisting B-cell clone might identify at-risk individuals.8
To focus on the relationship between treatment with JAK inhibitors and LPNs in patients with MPNs, Pemmaraju et al performed a large database review of 2583 patients with MPNs. They did not find a significant difference in the incidence of lymphoma when comparing patients who received JAK inhibitor therapy with those who did not. Only 9 (0.56%) of 1617 patients with myelofibrosis developed a lymphoma; among these 9 patients, 6 had previous treatment with JAK inhibitors and 3 did not; the P value was not statistically significant between the two groups (P = .082). Five (0.52%) of 966 patients with essential thrombocythemia or polycythemia vera developed lymphoma, but none of them had previously received JAK inhibitors (P = not significant). In the patients who had received treatment with JAK inhibitors, there was marked heterogeneity in lymphoma subtypes, including both high- and low-grade lymphomas (3 diffuse large B-cell lymphomas [DLBCLs], 2 mantle cell lymphomas, and 1 lymphoma of the scalp). This is in contrast with the observation of the Viennese group8 that reported a correlation between JAK inhibitors and aggressive B-cell lymphoma: all 4 patients in the Viennese cohort suffered from an aggressive B-cell lymphoma with extranodal involvement, including the bone marrow with a leukemic phase in 3 patients.
Pemmaraju et al suggested that the inconsistency between their results and those of the Viennese group might depend on the larger size of the data set, the longer duration of follow-up, the different treatment received for MPNs (none of their patients received pipobroman), and demographic factors (American vs European cohorts).
We thus have 2 contradictory articles: 1 showing that treatment with JAK inhibitors is associated with an increased risk for aggressive B-cell lymphoma and 1 demonstrating that there is no significant increase in lymphoma rates. What are the implications for clinical practice? Our professional suggestion is to perform flow cytometry immunophenotyping and analysis for immunoglobulin heavy chain variable region (IGHV) gene rearrangements, ideally on bone marrow or on peripheral blood, to detect the presence of a B-cell clone before starting ruxolitinib.
As shown in the figure, if IGHV is polyclonal, we would initiate treatment with JAK inhibitors with a policy of regular follow-up (green traffic light); if IGHV is monoclonal, hematologists should consider treatment with a JAK inhibitor and balance the risk-benefit ratio. So far, there is insufficient solid evidence for a particular recommendation, but 1 approach would be to monitor these patients with special attention for monoclonality during treatment follow-up. We do not have the tools to decide whether the treatment can be initiated with a warning (yellow traffic light) or if it is better to avoid using the treatment altogether (red traffic light). Generally only one-third of patients with DLBCL have bone marrow involvement at the time of diagnosis, and morphologic involvement of the peripheral blood with DLBCL is rare.9 Most of the patients with DLBCL in the Viennese cohort had bone marrow involvement (1 of them even had peripheral blood involvement), 1 patient had a BCL2 translocation, and another showed a clonal immunoglobulin gene rearrangement several months before lymphoma was diagnosed. All these factors raise the suspicion that these patients in fact had monoclonal B lymphocytosis or low-grade lymphoma that progressed to high-grade lymphoma during treatment with JAK inhibitors.
In light of the contradictory data that are currently available, more research is needed to establish whether patients receiving treatment with JAK inhibitors have an enhanced risk of developing lymphoma and, in the event that lymphoma is confirmed in additional validation studies, we need to know more about the risk estimates involved.