Abstract
Background
Our knowledge around the incidence of relapse and primary refractory disease in patients with diffuse large B-cell lymphoma (DLBCL) in the rituximab era is mostly derived from randomized controlled trials or specialized center cohorts presenting selected patient materials. With new therapies being evaluated and launched at diagnosis as well as in the relapsed/refractory setting there is a need for a better understanding of the response and outcome with current treatment strategies. Here we investigated the incidence of primary refractory DLBCL and first relapse as well as survival among patients with relapsed/refractory DLBCL in a population-based cohort.
Methods
Patients with a primary diagnosis of DLBCL in 2007-2014 were identified using the Swedish Lymphoma Register in 5 of 6 health care regions, and followed until October 31st 2017. Primary CNS lymphomas and primary mediastinal B-cell lymphomas were excluded. The register contains information about clinical characteristics, primary treatment, treatment response and relapse. Information regarding treatment response and progression/relapse was validated through medical chart review in all patients. Primary treatment with curative intent was defined as having started treatment with anthracycline-based chemotherapy (mostly R-CHOP). Early relapse was defined as having primary refractory disease (stable or progressive disease (SD/PD) at response evaluation) or relapse within a year from diagnosis. Overall and progression-free survival probabilities were estimated with the Kaplan-Meier method in the entire cohort and among relapsed/refractory patients by early/late relapse and by age +/- 70 years. Additionally, cumulative incidence of relapsed/refractory disease by follow-up time was shown graphically accounting for the presence of competing risks.
Results
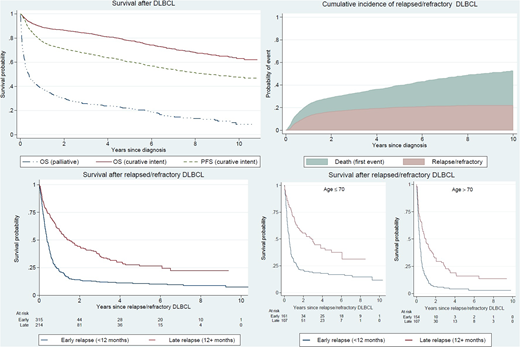
In the study population of 3165 patients, median age at diagnosis was 71 years (range 18 to 97). In this unselected cohort, 80% of the patients started treatment with curative intent. Five-year overall survival (OS) for patients treated with curative intent was 78% (95% CI: 76-80) and 5-year progression free survival (PFS) was 60% (95% CI: 58-62). Patients who received non-curative intent or palliative therapy (20%) had a median OS of 4.7 months (95% CI: 3.4-5.7). The 10-year cumulative incidence of relapsed/refractory disease in patients treated with curative intent was 22% (95% CI: 20-24) and the majority relapsed within two years (n=315, 60%). Five percent of the patients starting primary treatment with curative intent, only received 1 or 2 treatment cycles and were not evaluated for response.
Patients with relapsed/refractory disease had a poor prognosis. For patients with early relapse, median survival from the date of relapse was 4.6 months (95% CI: 4.1-5.7) and 5-year OS was 11% (95% CI; 8-15) whereas patients with late relapse had a median survival of 18.0 months (95% CI: 13.0-24.5) and a 5-year OS of 26% (95% CI: 20-33). Survival in younger patients (≤70 years) by early and late relapse was higher although still low; 5-year OS in early relapse was 17% (95% CI: 12-23) and in late relapse 37% (95% CI: 27-48). Among older patients (>70 years) with early relapse, 5-year OS was only 4% (95% CI: 2-9) and in patients with late relapse it was 16% (95% CI: 9-24). More information regarding treatment intensity among relapsed/refractory patients will be presented.
Conclusion
The 10-year cumulative incidence of relapsed/refractory disease in patients with DLBCL treated with curative intent is 22% in this population-based study, which is lower compared to previous reports. Outcome for patients with relapsed/refractory disease continues to be poor especially for patients with early progression/relapse, even among younger patients going on to intensive second-line chemotherapy aiming for autologous stem cell transplantation. These results underscore both the urgency of new therapies for relapsed/refractory DLBCL as well as the need for identification of high-risk patients already at diagnosis.
Figure 1. Overall survival (OS) and progression-free survival (PFS) among 3165 patients with diffuse large B-cell lymphoma (DLBCL) diagnosed in Sweden 2007-2014 treated with curative or palliative intent, and cumulative incidence of relapsed/refractory disease and survival among 529 relapsed/refractory patients by timing of relapse (early/late) and age (+/-70 years).
Harrysson:Janssen Pharmaceuticals: Other: The Department have recieved partial funding from Janssen Pharmaceuticals. Eloranta:Janssen Pharmaceuticals: Other: S Eloranta is currently employed as a project coordinator and her salary is funded via a public-private real world evidence collaboration between Karolinska Institutet and Janssen Pharmaceuticals. Ekberg:Janssen Pharmaceuticals: Other: The department has received partial funding from Janssen Pharmaceuticals. Wahlin:Gilead: Consultancy, Honoraria, Research Funding; Roche: Research Funding. Smedby:Janssen Pharmaceuticals: Other: The Department have recieved partial funding from Janssen Pharmaceuticals.
Author notes
Asterisk with author names denotes non-ASH members.