Abstract
INTRODUCTION
It is unknown whether chimeric antigen receptor (CAR)-T cells expand or provide clinical benefit in patients (pts) without detectable disease before infusion. JULIET (NCT02445248) is a single-arm, open-label, multicenter, global, phase 2 trial investigating tisagenlecleucel, an autologous anti-CD19 CAR-T cell therapy recently approved by the US Food and Drug Administration for treatment of adult pts with r/r DLBCL. Pts were enrolled in JULIET based on local assessment of measurable disease at screening. To control disease and stabilize pts during the period between apheresis and tisagenlecleucel infusion, most pts (92%) received bridging chemotherapy of the treating physician's choice. Bridging chemotherapy is unlikely to provide sustained clinical response because currently observed rates of response (complete response [CR], 8%; partial response [PR], 18%) and overall survival (OS; median, 4.4 mo) are poor with conventional treatments (Crump et al. Blood. 2017.). A subset of pts in JULIET achieved CR and had no measurable disease remaining by positron emission tomography (PET) following bridging therapy and received tisagenlecleucel thereafter per protocol. Assessment of CAR-T cell expansion and outcomes in this pt population were the purposes of this analysis.
METHODS
This is an exploratory post-hoc analysis of 8 pts (of 111 from JULIET [data cutoff, December 8, 2017]) who had a CR after bridging chemotherapy and before tisagenlecleucel infusion. Staging at the baseline scan before tisagenlecleucel infusion showed no evidence of active disease per an independent review committee (IRC), with PET 5-point scale = 1. Of note, 2 pts had evidence of nonindex lesions on baseline CT scans. The protocol mandated efficacy assessments at 1, 3, 6, 9, 12, 18, 24, 36, 48, and 60 months.
RESULTS
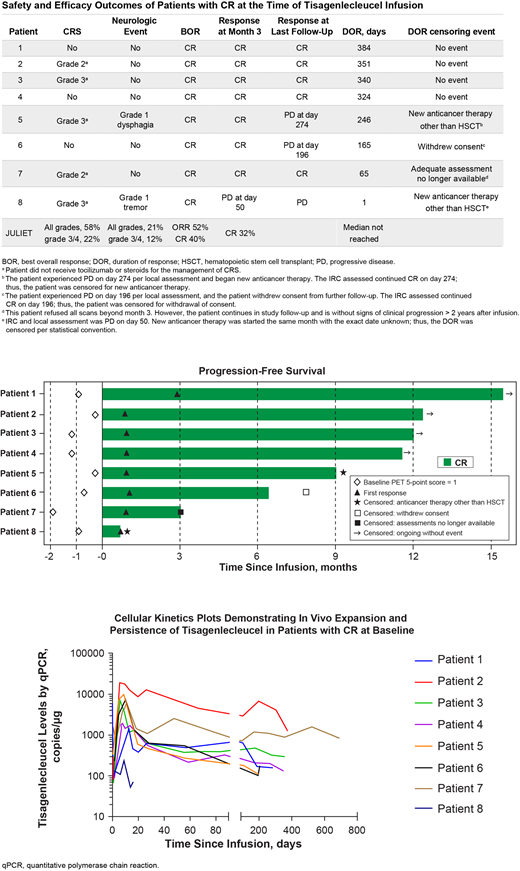
The 8 pts with a CR before tisagenlecleucel infusion were aged 54 to 71 years (3 were > 65 years); 6 were women. The proportion of pts with transformed follicular lymphoma (25%) was similar to that of the overall JULIET study population (22%). At study entry, 3 pts had stage I disease, 2 stage III, and 3 stage IV. Seven of 8 had relapsed disease (CR/PR to last therapy and subsequent progression before enrollment); 1 was refractory (progressive or stable disease as best response to last therapy before enrollment). Six pts had received 2 prior lines of therapy and 2 had received 3 prior lines; 4 had previously undergone a stem cell transplant.
Five of 8 pts (63%) experienced cytokine release syndrome (CRS; 2 grade 3, 3 grade 2 using the Penn scale); 2 pts experienced neurological events (both grade 1). No pts received tocilizumab or steroids for CRS management. In JULIET, 58% of pts experienced CRS (22% grade 3 or 4) and 21% neurological events (12% grade 3 or 4) within 8 weeks of infusion. One of the 8 pts had grade 2 cytopenia ongoing at day 28, 6 had infections (1 grade 2, 4 grade 3, 1 grade 4), and 2 had febrile neutropenia (1 grade 3, 1 grade 4).
Tisagenlecleucel cells expanded rapidly over the first 28 days in 7 of 8 pts, and transgene levels were measurable for up to 400 days after infusion. The pt who did not show a high degree of expansion and persistence relapsed at day 50.
All 8 pts were in CR 28 days after infusion; 7 of 8 were in CR at month 3; 5 of 8 remained on study and were progression free > 12 months after infusion. At data cutoff, the following responses were observed in these 8 pts: 4 remained in remission; 1 pt alive was without clinical signs of relapse but declined imaging beyond month 3; 1 withdrew consent after locally assessed relapse on day 196; 1 relapsed at day 50 and died of chronic kidney disease 109 days after tisagenlecleucel infusion; and 1 had locally assessed relapse at day 274, received additional anticancer therapies for disease progression, and died of DLBCL 544 days after CAR-T infusion.
CONCLUSIONS
Despite no measurable disease remaining on preinfusion imaging, CAR-T cell expansion was observed in 7 of 8 pts in this setting, similar to the expansion in adults with active r/r DLBCL. Five of the 8 pts remained on study and were progression free > 12 months after infusion, which is higher than would be estimated with bridging chemotherapy alone. These data provide preliminary evidence that CAR-T cells provide clinical benefit in pts with r/r DLBCL who achieve CR with subsequent therapies and for design of CAR-T cell trials as consolidative therapy.
Bishop:United Healthcare: Employment; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Speakers Bureau; Juneau Therapeutics: Speakers Bureau; Novartis Pharmaceuticals Corporation: Speakers Bureau. Maziarz:Athersys, Inc.: Patents & Royalties; Kite Therapeutics: Honoraria; Juno Therapeutics: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Novartis Pharmaceuticals Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Waller:Celldex: Research Funding; Cambium Medical Technologies: Consultancy, Equity Ownership; Kalytera: Consultancy; Novartis Pharmaceuticals Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Other: Travel Expenses, EHA, Research Funding. del Corral:Novartis Pharmaceuticals Corporation: Employment. Tiwari:Novartis Healthcare Private Limited: Employment. Anak:Novartis Pharma AG: Employment. Awasthi:Novartis Institutes for Biomedical Research: Employment; Celgene: Equity Ownership; Exelixis: Equity Ownership. Romanov:Novartis Pharmaceuticals Corporation: Employment. Schuster:Gilead: Membership on an entity's Board of Directors or advisory committees; Genentech: Honoraria, Research Funding; Merck: Consultancy, Honoraria, Research Funding; Novartis Pharmaceuticals Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Dava Oncology: Consultancy, Honoraria; Physician's Education Source, LLC: Honoraria; OncLive: Honoraria; Nordic Nanovector: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.