Abstract
Background: Diffuse large B cell lymphoma (DLBCL) is the most frequent lymphoma subtype with up to two thirds of patients achieving cure following standard immuno-chemotherapy. However, approximately 10-15% will be primary refractory and a further 20-30% relapse. There is an urgent need for improved biomarkers to identify these poor risk patients at diagnosis who are destined to fail standard therapy so that novel approaches can be considered. The diagnostic peripheral blood monocyte count is known to be predictive of outcome in DLBCL, with higher counts identifying patients with worse outcome but mechanistic understanding is currently lacking. The aim of this study was to investigate the peripheral blood monocyte population driving this phenomenon and we present here a deep phenotypic analysis of the monocyte compartment at diagnosis in patients with extremes of outcome following immuno-chemotherapy.
Methods: We identified 112 immuno-chemotherapy treated DLBCL patients with viably cryopreserved peripheral blood mononuclear cells (PBMCs) in our tissue bank. The diagnostic absolute monocyte (AMC) and lymphocyte counts (ALC) were examined as prognostic variables. For phenotypic analysis a subset of 20 patients, 10 with relapse/refractory disease within 12 months of therapy (Poor risk) and 10 with continued complete remission >24 months post therapy (Good risk), were selected. PBMCs from these patients were stained with a panel of 29 metal-tagged antibodies designed to identify immune populations and characterize the monocyte compartment and analyzed using cytometry by time-of-flight (CyTOF2TM). Samples were acquired in batches run with the same healthy donor control PBMCs to ensure consistency of staining. Normalized data were subjected to traditional Boolean gating in Cytobank (www.mrc.cytobank.org) to identify CD45+ live single cells and further analyzed using FlowSOM and CITRUS.
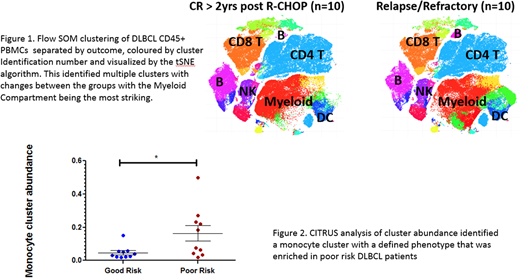
Results: In the full 112 patient cohort, a higher diagnostic AMC (≥0.6 x 109/L) predicted worse outcome (5-year OS, 59% vs 74%, p=0.047*). The lymphocyte to monocyte ratio (LMR) was more discriminatory, with a low LMR (<2.6) predicting worse outcome (5-year OS, 49% vs 81%, p=0.0009*). CyTOF analysis of the CD45+ events from all 20 DLBCL patients were clustered in an unsupervised manner with FlowSOM and visualized according to the tSNE algorithm. This identified all expected immune populations with further subdivision into multiple clusters / sub-populations based on protein marker expression. Repeating the FlowSOM analysis by outcome revealed clusters with clear differences between the 2 groups, including striking changes to the monocyte compartment (Figure 1). CITRUS, an algorithm for automated biomarker discovery, was used to further assess the differences in the monocyte compartments between poor risk and good risk DLBCL patients. This identified a monocyte subset, representing approximately 10% of non-lymphoid CD45+ PBMCs, with increased abundance in poor risk patients with relapsed/refractory disease (p=0.0028*, Figure 2). This subset expressed markers including HLA-DR, CD11b, CD13, CD14, CD33, SIRPα (CD172a), CCR2 (CD192, monocyte chemo-attractant protein receptor), CD206 and PD-L1 (CD274).
Conclusions: We confirmed the previous finding that the diagnostic AMC is predictive of outcome in our cohort of DLBCL patients and have identified differences in the peripheral blood monocyte compartment between patients with good and poor risk disease. Unsupervised analysis using FlowSOM identified monocyte clusters with greater abundance in poor risk patients. A second high-dimensional single cell data analysis algorithm, CITRUS validated this finding by revealing a stratifying monocyte sub-population that was enriched in poor risk patients. This phenotype of peripheral blood monocytes may account for the adverse nature of a higher AMC at diagnosis. We are now functionally characterizing these monocytes and evaluating their relationship to differences between the lymphoid populations of good and poor risk patients.
Seymour:Celgene: Research Funding. Gribben:Janssen: Honoraria, Research Funding; Pharmacyclics: Honoraria; Abbvie: Honoraria; Kite: Honoraria; Acerta Pharma: Honoraria, Research Funding; Wellcome Trust: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; NIH: Research Funding; Unum: Equity Ownership; Roche: Honoraria; Novartis: Honoraria; Cancer Research UK: Research Funding; Medical Research Council: Research Funding; TG Therapeutics: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.