Abstract
Introduction: PTPN11 encodes the protein tyrosine phosphatase SHP 2, which relays signals from growth factor receptors to RAS and other effectors. Germline and somatic mutations in PTPN11 are well described in the pediatric population and associated with Noonan Syndrome and Juvenile Myelomonocytic Leukemia (JMML). Pathogenesis of JMML specifically appears to be through activation of the RAS-RAF-MAP kinase pathway leading to dysregulated myeloid differentiation. There are also data to suggest that somatic PTPN11 mutations portend a poor prognosis in MDS patients (pts) receiving hypomethylating agents. The significance of PTPN11 when sporadically mutated in adults with AML remains controversial as several analyses have thus far failed to show any clinical relevance. This study evaluated the clinical significance of somatic PTPN11 mutations in a single center cohort.
Methods: From 7/2015-7/2018, data from an AML database at New York Presbyterian/Weill-Cornell Medical Center was queried for the presence or absence of mutations in the PTPN11 gene as well as on all pts with TP53 mutations to use as a surrogate, given its well-known status as a poor prognostic factor. Log-rank tests were used to compare survival data, while Fisher-exact test was used to compare non-survival data (i.e. CR rates). For multivariate analysis, linear regression was performed and looked at mutational status, age, cytogenetics (CG), and controlled for age and European Leukemia Net (ELN) risk.
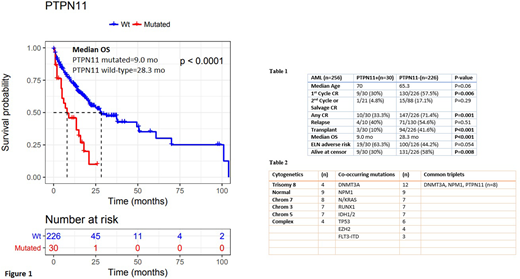
Results: 256 AML pts with complete evaluable data. 30 were found to harbor mutations in PTPN11 at diagnosis. Within the PTPN11 mutated cohort, median age was 70, 15 were female and 15 were male. 1st cycle complete response (CR) rate was 30% (9/30) and one additional pt (4.8%) achieved a salvage CR. Hematopoietic stem cell transplantation (HSCT) was provided to 3/30 (10%) and of those, 1/3 (33.3%) relapsed, within 8 months. In the pts who achieved a CR, 4/10 (40%) relapsed. Median overall survival (OS) of the PTPN11 mutated cohort was 9 months (mo). Four patients (13.3%) are alive and in a CR >6 mo at time of censor.
DNMT3A, NPM1, K/NRAS, RUNX1, TP53 and IDH1/2 were commonly co-mutated (n=12,9,7,7,6, and 6 respectively, table 2) with PTPN11 mutations. DNMT3A, NPM1 and PTPN11 were commonly mutated together in pts, n=8 (26.7%). The PTPN11 mutation was a single mutation in 2 pts. Common CG findings include normal (n=9), complex (n=4), trisomy 8 (n=4), chr. 3 abnormalities (n=7), chr. 5 (n=7) and chr. 7 (n=8).
When comparing the PTPN11 mutated pts to all AML pts diagnosed at this center during the same time period without a PTPN11 mutation (table 1), 1st cycle CR rate (30% vs 57.5%, p=0.006), any CR (33.3% vs 71.4%, p=0.001), HSCT (10% vs 41.6%, p<0.001), Median OS (9.0 mo vs 28.3 mo, log-rank p,<0.001, figure 1) and proportion of pts alive at censor (30% vs 58%, p=0.008) were all significantly different between the two groups. Neither choice of initial induction regimen (proportion of high dose cytarabine based therapy) nor proportions of pts with adverse risk AML by ELN differed between the two groups (46.7% vs 48.2%, p=0.86 and 63.3% vs 44.2, p=0.054). Numbers were too small to compare relapse free survival, however, relapse rates were not significantly different.
In a multivariate analysis of the full cohort of 256 pts, PTPN11,TP53 and Age were all independently associated with increased risk of death compared to the full cohort, with a HR of 2.00, CI 1.16-3.44 p=0.01, HR 1.9, CI 1.04-3.46, p=0.04, HR 1.05, CI 1.03-1.07, p<0.001, respectively.
We also compared the OS of PTPN11 mutated AML to TP53 mutated AML and found that while there was a small difference in median OS (9.0 mo vs 9.8 mo) it was not significant, p=0.77.
Discussion: This comparison of PTPN11 mutant to PTPN11 wild-type AML is the largest single center analysis and the first to show a significant chemotherapy response and survival difference that is similar to AML with a TP53 mutation. The multivariate analysis showed PTPN11 carried a poor prognosis (HR for death of 2.00). Mutations in DNMT3A and NPM1 with PTPN11 was common in our cohort, confirming previous work.
Conclusion: These data suggest that the presence of PTPN11 is associated with an aggressive disease with poor outcome and treatment resistance. Pre-clinical investigation has been initiated to explore a mechanistic role for these clinical findings, with the hope of testing novel therapeutics on an animal model of AML with PTPN11 mutations.
Roboz:Cellectis: Research Funding; Daiichi Sankyo: Consultancy; Eisai: Consultancy; Celltrion: Consultancy; Bayer: Consultancy; Sandoz: Consultancy; Janssen Pharmaceuticals: Consultancy; Celltrion: Consultancy; Celgene Corporation: Consultancy; Otsuka: Consultancy; Janssen Pharmaceuticals: Consultancy; Pfizer: Consultancy; Roche/Genentech: Consultancy; Argenx: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; AbbVie: Consultancy; Aphivena Therapeutics: Consultancy; Cellectis: Research Funding; Celgene Corporation: Consultancy; Eisai: Consultancy; Jazz Pharmaceuticals: Consultancy; Sandoz: Consultancy; Astex Pharmaceuticals: Consultancy; Astex Pharmaceuticals: Consultancy; Argenx: Consultancy; Orsenix: Consultancy; Bayer: Consultancy; AbbVie: Consultancy; Otsuka: Consultancy; Jazz Pharmaceuticals: Consultancy; Novartis: Consultancy; Aphivena Therapeutics: Consultancy; Daiichi Sankyo: Consultancy; Orsenix: Consultancy; Roche/Genentech: Consultancy. Lee:AstraZeneca: Consultancy; Clinipace: Consultancy; Karyopharm Therapeutics Inc: Consultancy; LAM Therapeutics: Research Funding; Amgen: Consultancy. Desai:Argenx: Consultancy; Cellerant Inc: Consultancy. Guzman:Cellectis: Research Funding. Ritchie:Incyte: Consultancy, Speakers Bureau; NS Pharma: Research Funding; Bristol-Myers Squibb: Research Funding; Astellas Pharma: Research Funding; ARIAD Pharmaceuticals: Speakers Bureau; Novartis: Consultancy, Other: Travel, Accommodations, Expenses, Research Funding, Speakers Bureau; Pfizer: Consultancy, Research Funding; Celgene: Consultancy, Other: Travel, Accommodations, Expenses, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.