Abstract
Myeloproliferative neoplasms (MPN) are most common in old people (>60 years) and are rarely identified in children and young adults where information about complication rates and long-term data are lacking. To improve our knowledge, we retrospectively collected cases of young patients diagnosed with MPN before 25 years of age and analysed data of their disease to date, including vascular events and disease evolution.
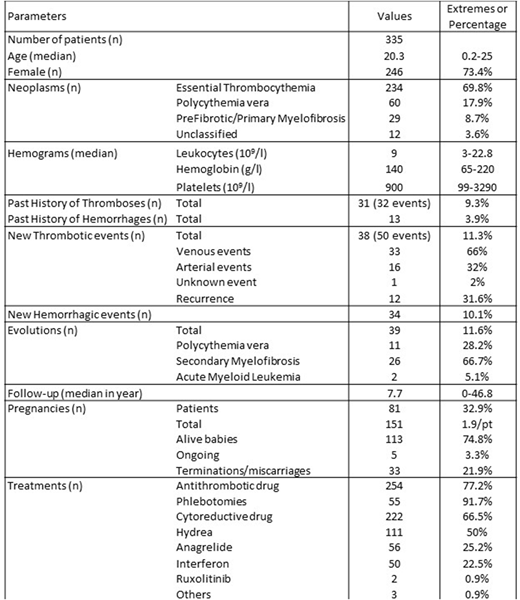
Data were collected in 29 hospital centres from 12 countries. Between 1971 and 2018, 335 young patients were diagnosed for an MPN before the age of 25. They were mostly females (n=246; 73.4%) with a median age of 20.3 years at diagnosis (2.5 months-25 years). Essential thrombocythemia was diagnosed in 234 patients (69.8%), polycythemia vera in 60 (17.9%) and myelofibrosis or unclassified MPN in 41 (12.3%).
Most of the diagnoses were made following a coincidental blood count analysis (n=75; 51%) some based on symptoms (n=57; 38.8%) or thrombotic events (n=15; 10.2%). In terms of complications before or at diagnosis, 31 (9.3%) patients experienced thrombosis, mostly venous (75%) and 13 (3.9%) had hemorrhage. At diagnosis, the median leukocyte count was 9x109/l (range: 3-22.8), median hemoglobin count 140 g/l (65-220) and median platelet count 900x109/l (99-3290). To assess the diagnosis, 158 patients (47.2%) had had bone marrow aspirates and 214 (63.9%) a bone marrow biopsy. Mutational status was available in 319 (90%) cases: 194 (60.8%) were JAK2V617F positive, 48 (15%) had a calreticulin mutation, 76 (23.8%) were triple-negative and 1 patient had MPL mutation.
The median follow-up of the cohort was 7.7 years (0-46.8) with 134 patients (40%) having follow-up for more than 10 years. 81 female patients (32.9%) experienced pregnancies. During this period, 295 patients (88%) received at least one drug for their MPN: 254 patients (77.2%) received antithrombotic drug and 222 patients (66.5%) a cytoreductive drug. As first line of treatment, hydroxycarbamide was given to 111 patients (50%) whereas anagrelide was given to 56 patients (25.2%) and interferon to 50 (22.5%).
During the follow-up, 97 patients (29%) experienced at least one complication. In terms of cardiovascular events, 38 (11.3%) patients experienced thromboses with 50 events in total (recurrences in 12 cases), including 33 venous events (66%) of which 15 were localized in the splanchnic territory (45.5%). Hemorrhagic events were recorded in 34 cases (10.1%). During the follow-up, 39 patients (11.6%) have evolved: 11 from ET to PV (28.2%), 26 into MF (66.7%) and 2 into AML (5.1%). All evolutions were exclusive: one event per patient. At the time of the analysis, 66 patients (19.7%) were declared as lost of follow up and 4 were dead (1.2%).
This is the largest cohort of patients aged below 25 years at the time of diagnosis demonstrates that despite their youth most of them (88%) received drug(s) for the management of their MPN. There was a high incidence of complications (29%), with vascular events and disease evolution occurring at equal frequency although death was uncommon (1.2%). Rates of events were disease evolution: 1.51/100pts/y; thromboses: 1.47 and hemorrhages: 1.32. No specific national or international guidance exists for MPN patients of this age; but our data suggest that these are not benign conditions and patients need to be carefully followed and treated.
Kiladjian:Celgene: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; AOP Orphan: Membership on an entity's Board of Directors or advisory committees, Research Funding. Giraudier:Novartis: Research Funding. Griesshammer:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Harrison:Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Speakers Bureau; Roche: Consultancy, Honoraria; Gilead: Honoraria, Speakers Bureau; CTI BioPharma: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.