Abstract
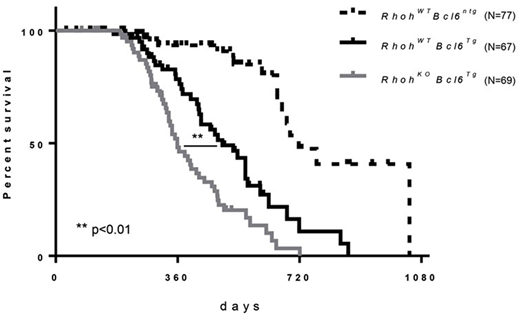
RHOH encodes a GTPase-deficient, hematopoietic-specific small GTPase first identified as a hypermutable gene in DLBCL (Pasqualucci et al. 2001). RhoH is critical for T cell receptor signaling and Rhoh-deficient (RhohKO) mice have T cell lymphopenia (Gu et al., 2006) and loss of function mutations of RHOH are associated with Epidermodysplasia Verruciformis (Crequer et al., 2012). However, the role of RhoH in the biology of DLBCL is still unknown and its role in B lymphoid development is incompletely studied. We investigated the role of RhoH in normal germinal center formation and in a murine model of DLBCL by crossing RhohKO mice with Iµ-HABcl-6 transgenic (Bcl-6Tg) mice (Cattoretti G, et al., 2005). In young RhohKOmice, deficient development of CXCR5+ follicular T helper (Tfh) cells results in defective germinal center (GC) formation and impaired immunoglobulin switching in vivo. In spite of this defect in GC formation, RhohKO; Bcl-6Tg (KOTg) mouse demonstrated accelerated lymphoma progression associated with larger spleens and significantly earlier death (Log-rank test p<0.01, Figure 1). Immunohistochemistry data suggested increased expression of IRF-4 and enhanced expression of BCL-6 in KOTg mice, findings confirmed by immunoblot and consistent with an activated B-cell (ABC)-DLBCL phenotype. To analyze the mechanism underlying these results, B cell lymphoma cell lines from KOTg lymphoma mice were established. Multiple attempts to establish RhohWT lymphoma cell lines failed, although we also successfully established a lymphoma cell line from RhohKO; Bcl-6(ntg) (KONtg) mice. Re-expression of RhoH in these lines via retrovirus mediated gene transfer led to significantly decreased proliferation (5.9x106±9.6x105 cells vs 8.6x106±9.6x105 cells after 5-days culture; KOTg vs KOTg-RhoH, mean±SEM, p<0.05) that was associated with clear reduction in BCL-6 expression. These data suggest that BCL-6 is a direct or an indirect transcriptional target of RhoH. Our laboratory previously reported that KAISO, a dual-specific, Broad complex, Trantrak, Bric-a-brac/Pox virus, Zinc finger (POZ-ZF) transcription factor interacts and colocalizes with RhoH in the nucleus, whereas knockdown of RhoH inhibits the nuclear localization of KAISO in Jurkat cells (Mino A, et al., 2016). In addition, Kaiso has been shown to be a key regulator of spleen germinal center formation by repressing Bcl-6 expression in splenocytes (Koh D, et al., 2013). We hypothesized that the deletion of Rhoh may lead to the decreased nuclear localization of KAISO and result in increased the expression of Bcl-6. We first confirmed that RhoH bound KAISO in RhoH-transduced KO lymphoma cells by co-immunoprecipitation. Further immunoblot analysis and quantitative PCR (qPCR) demonstrated decreased BCL-6 expression in lymphoma cells in which RhoH was re-expressed (KOTg-RhoH and KONtg-RhoH) compared with empty vector-transduced lymphoma cell lines. Interestingly, p53 a BCL-6 target was increased in RhoH-transduced lymphoma cell lines. These data indicate that RhoH affects BCL-6 expression in B cell lymphoma cell lines and suggest that RhoH may be involved in DLBCL development by co-regulating BCL-6 expression affecting downstream targets via interaction with KAISO.
Williams:Bluebird Bio: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.