Abstract
Background: CPX-351 (Vyxeos®) is a dual-drug liposomal encapsulation of cytarabine and daunorubicin at a synergistic ratio. In a large randomized, open-label, multicenter, phase 3 study of CPX-351 versus conventional cytarabine/daunorubicin chemotherapy (7+3 regimen) in adults aged 60-75 years with newly diagnosed high-risk/sAML, patients treated with CPX-351 had significantly longer survival times and higher remission rates (Lancet JE, et al. J Clin Oncol. 2018). Based on these results, CPX-351 was approved by the US FDA in 2017 for the treatment of adults with newly diagnosed therapy-related AML or AML with myelodysplasia-related changes (AML-MRC). This abstract reports the results of an EAP study that provided expanded access to CPX-351 for older patients who met the eligibility criteria for the phase 3 study and collected additional data on safety and efficacy.
Methods: In this phase 4, single-arm, open-label EAP, patients were between 60-75 years of age and had confirmed high-risk/sAML (therapy-related AML [tAML], AML with a history of myelodysplasia [MDS] or chronic myelomonocytic leukemia [CMML], or de novo AML with MDS karyotype). Patients could receive up to 2 cycles of induction with CPX-351 100 units/m2 (cytarabine 100 mg/m2 + daunorubicin 44 mg/m2) on Days 1, 3, and 5 (2nd induction: Days 1 and 3). Patients with complete remission (CR) or CR with incomplete platelet or neutrophil recovery (CRi) could receive up to 4 cycles of consolidation with CPX-351 65 units/m2 (cytarabine 65 mg/m2 + daunorubicin 28.6 mg/m2) on Days 1 and 3. The primary endpoint was safety, and the secondary endpoint was the rate of CR+CRi.
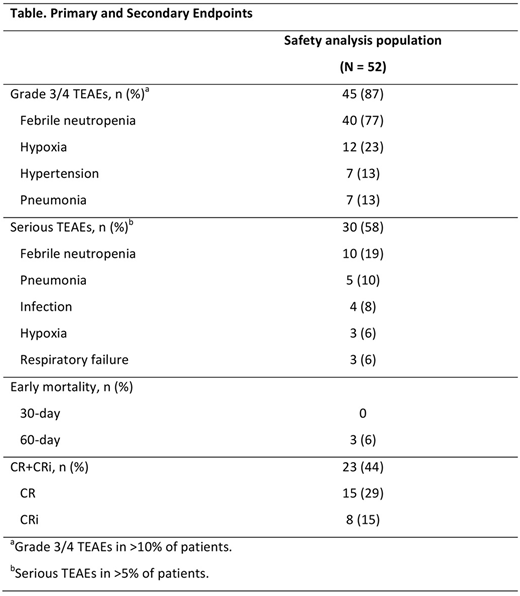
Results: Overall, 52 patients received ≥1 dose of CPX-351 and were included in the safety analysis. Among these patients, the median age was 70 years (range: 55-75) and 23% had an Eastern Cooperative Oncology Group score of 2. Median time since diagnosis was 0.30 months (range: 0.03-36.14) months. Patients with AML-MRC accounted for 77% of the safety analysis population, including those with antecedent MDS with prior hypomethylating agent (HMA) treatment (25%), antecedent MDS without prior HMA treatment (21%), antecedent CMML (8%), and de novo AML with MDS karyotype (23%); 23% of patients had tAML. All patients received 1 induction cycle and 25% received 2 induction cycles; 17%, 8%, 4%, and 2% of patients received 1, 2, 3, and 4 consolidation cycles, respectively.
CR+CRi was achieved in 23 patients (44% [95% CI: 31%, 59%]), including 15 with CR (29% [95% CI: 17%, 43%]) and 8 with CRi (15% [95% CI: 7%, 28%]; Table). The median time to remission was 37 days (range: 15-72). At the end of the study, 47 (90%) of patients were still alive and 11 (21%) patients received transplant.
All patients were alive at Day 30, and the mortality rate at Day 60 was 6%. The safety profile observed in this EAP study was consistent with that of the phase 3, randomized study (Table). Treatment-emergent adverse events (TEAEs) of any grade occurred in 96% of patients, including 44% of patients with an TEAE deemed related to treatment; the only treatment-related AE that occurred in >10% of patients was febrile neutropenia (31%). Only 2 patients (4%) discontinued treatment due to an AE (ejection fraction decrease and intercranial hemorrhage [n = 1 each]). Five patients (10%) had grade 5 AEs during the study, including disease progression, multiple organ dysfunction syndrome, acute respiratory distress syndrome, aspiration, and intracranial hemorrhage (n = 1 each).
Conclusions: The data from this EAP study were consistent with results from the randomized, phase 3 study. The safety profile in the EAP study was similar to that observed in the phase 3 study and there was a similar CR+CRi rate (44% vs 48%, respectively) in this high-risk/sAML population.
Roboz:Sandoz: Consultancy; Cellectis: Research Funding; Argenx: Consultancy; Bayer: Consultancy; Daiichi Sankyo: Consultancy; Novartis: Consultancy; Aphivena Therapeutics: Consultancy; Sandoz: Consultancy; AbbVie: Consultancy; Pfizer: Consultancy; Roche/Genentech: Consultancy; Novartis: Consultancy; Celgene Corporation: Consultancy; Celltrion: Consultancy; Jazz Pharmaceuticals: Consultancy; Roche/Genentech: Consultancy; Eisai: Consultancy; Otsuka: Consultancy; Astex Pharmaceuticals: Consultancy; Celgene Corporation: Consultancy; Janssen Pharmaceuticals: Consultancy; Janssen Pharmaceuticals: Consultancy; Eisai: Consultancy; Otsuka: Consultancy; Orsenix: Consultancy; Celltrion: Consultancy; AbbVie: Consultancy; Daiichi Sankyo: Consultancy; Aphivena Therapeutics: Consultancy; Bayer: Consultancy; Argenx: Consultancy; Jazz Pharmaceuticals: Consultancy; Astex Pharmaceuticals: Consultancy; Cellectis: Research Funding; Orsenix: Consultancy; Pfizer: Consultancy. Rubenstein:Alexion: Consultancy, Honoraria, Speakers Bureau; Cyclacel: Other: Travel support; Astex: Other: Travel support. Schiller:Celator/Jazz Pharmaceuticals: Research Funding; Pharmacyclics: Research Funding. An:Jazz Pharmaceuticals: Employment. Mancino:Jazz Pharmaceuticals: Employment. Chiarella:Celator/Jazz Pharmaceuticals: Employment, Equity Ownership. Louie:Celator/Jazz Pharmaceuticals: Employment, Equity Ownership, Patents & Royalties. Lin:Jazz Pharmaceuticals: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.