TO THE EDITOR:
Cold agglutinin disease (CAD) is a chronic hemolytic disorder caused by anti–red blood cell immunoglobulin M (IgM) autoantibodies most often monoclonal with k light-chain restriction.1,2 The autoantibody reacts at temperatures lower than the body temperature, causing autoagglutination and complement-dependent red blood cell destruction. CAD is a rare disease, accounting for ∼15% of autoimmune hemolytic anemias.3,4 Although clinical manifestations (acrocyanosis, Raynaud phenomenon, and anemia) can be mitigated by avoiding cold exposure, the median hemoglobin level in unselected CAD patients is 89 g/L.5 Moreover, CAD is a relapsing disease, requiring transfusions and multiple therapies to obtain disease control.6
There are few therapies for symptomatic CAD patients. Both corticosteroids and immunosuppressants are less effective in CAD than in warm autoimmune hemolytic anemias.7,8 Rituximab is presently considered the standard treatment; however, the response rate is ∼50%. Responses are almost exclusively partial, and their median duration is only 12 months.9-11 Its efficacy can be significantly increased and prolonged by the association with fludarabine or bendamustine, which, however, also adds the common side effects of these chemotherapeutic agents in patients without a clinically overt neoplastic disorder.12-14
Bortezomib is a proteasome inhibitor that has been successfully used in the treatment of immunoglobulin-producing diseases, including multiple myeloma, light-chain amyloidosis, and Waldenstrom macroglobulinemia.15 The presence of an underlying B-cell clonal disorder in most cases of CAD3,16 together with the favorable effect of few infusions of bortezomib reported in 2 patients with refractory CAD17 prompted us to investigate the potential benefit of a short course of bortezomib in anemic CAD patients refractory or relapsed after previous treatments.
A prospective multicenter, phase 2, open-label study was conducted within the GIMEMA (Gruppo Italiano Malattie Ematologiche dell’Adulto) and registered at ClinicalTrials.gov (NCT01696474). It was approved by the Ethical Committees of participating centers. Data were collected and managed using REDCap electronic data capture.18
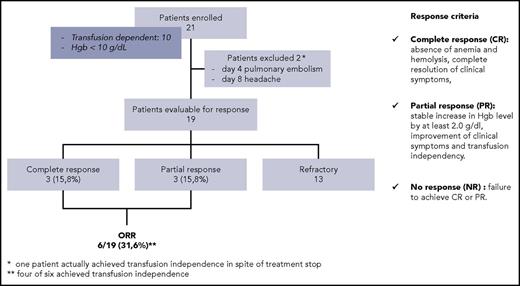
The primary objective of the study was the achievement of transfusion independence or at least a significant rise (>20 g/L) in hemoglobin. Secondary objectives were safety, duration of hematologic response, and effect on underlying B-cell disorders, if present. Diagnosis of CAD required the presence of chronic hemolysis and the detection of cryoagglutinins at 4°C (direct antiglobulin test strongly positive for complement C3 and negative/weakly positive for IgG). Other inclusion criteria were patients refractory or relapsed after at least 1 previous treatment, and hemoglobin concentration <100 g/L determined at least monthly during the 2 months before entering the trial. Exclusion criteria were concomitant clonal B-cell disorder requiring specific treatment, preexisting peripheral neuropathy, documented hypersensitivity to bortezomib, pregnancy, or psychiatric disorders. Twenty-one patients have been enrolled by 6 centers between April 2012 and March 2016. Eligible patients received a single course of bortezomib (1.3 mg/sqm IV on days 1, 4, 8, 11). Prophylaxis of herpes zoster reactivation was given with oral acyclovir (400 mg twice daily) until 1 month after the end of bortezomib. The response criteria were the same previously published.12,19
A summary of baseline characteristics of patients is provided in Table 1, and results achieved after bortezomib administration are summarized in Figure 1. Among the 19 evaluable patients, 3 achieved a complete response (15.8%) and 3 achieved a partial response (15.8%), for an overall response rate of 31.6%, including transfusion independence in 4 patients. Response was not correlated to previous treatments, including rituximab, nor to the presence of B-cell clonal disorder.
Flowchart of patients enrolled. Response was evaluated at 3 months after treatment start. Hgb, hemoglobin; ORR, overall response rate.
Flowchart of patients enrolled. Response was evaluated at 3 months after treatment start. Hgb, hemoglobin; ORR, overall response rate.
Four of 6 responding patients (66.7%; 95% confidence interval 37.9-100) maintained the response after a median follow-up of 16 months (range 10-31). One patient relapsed after >1 year and obtained a second remission after a second course of bortezomib. All patients were alive at last follow-up except 1 who died of septic shock during off treatment follow-up, 10 months after study entry. Median hemoglobin levels (g/L) before and after treatment were 87 and 96 in the whole cohort, 85 and 114 in responding patients, and 88 and 92 in nonresponding patients, respectively. The level of monoclonal immunoglobulin remained stable after treatment in 10/13 cases and decreased to <50% of pretreatment levels in 3 patients. The marrow B-cell lymphoid infiltrate persisted in 3 of 5 patients, decreased by >50% in 1 case, and became undetectable in 1. In 4 patients, bone marrow biopsy was not repeated. No correlation was found between the response to bortezomib and any of the pre- and the posttreatment characteristics of patients.
Treatment-related toxicity, including neurotoxicity, was rarely detected. A total of 19 adverse events of any grade were reported. Among 13 grade 1 to 2 adverse events, 1 was considered related to study drug (anemia grade 2). Six grade 3 to 4 adverse events were detected (headache, diarrhea, increased bilirubin levels, anemia, pulmonary embolism), among which only 1 (upper respiratory tract infection) was considered related to study drug.
There is an active search for novel therapies in symptomatic patients with CAD, to overcome the relatively low efficacy of rituximab and the potential toxicity of cytostatic agents like fludarabine and bendamustine, which currently represent state-of-the-art treatments. Therapies designed to target complement-mediated hemolysis of CAD are being actively investigated. Eculizumab has been reported effective in reducing transfusion requirement in preliminary reports,20,21 although in steady-state CAD most of hemolysis is not intravascular and C5 mediated. Moreover, in paroxysmal nocturnal hemoglobinuria eculizumab has been reported to increase C3b-mediated extravascular hemolysis,22 which is assumed to predominate in CAD. Thus, its efficacy in CAD needs to be confirmed in larger studies. More recently, the humanized antibody BIVV009 specific for the complement subunit C1s was proven effective in blocking the classical complement pathway activation and the resulting extravascular hemolysis both in vitro and in a phase 1 study.23,24 The antibody has received US Food and Drug Administration Breakthrough Therapy designation, and pivotal trials are currently ongoing (ClinicalTrial.gov: #NCT03347396; #NCT03347422).
Bortezomib has proven very effective in B-cell clonal lymphoproliferative disorders, characterized by the presence of a monoclonal IgM (eg, Waldenstrom macroglobulinemia), like in most cases of primary CAD. In the present study, a brief course of bortezomib obtained an overall response and a CR rate of 32% and of 16%, respectively, in patients with CAD. These results represent a proof of concept of the activity of bortezomib in a proportion of patients with CAD, which had been suggested to date only by case reports of single patients.17 Treatment was well tolerated, and toxicity was limited. All patients had received prior treatments, including rituximab and cytostatic agents, further supporting the efficacy of bortezomib. Moreover, despite the short duration of treatment, two-thirds of responding patients enjoyed a long-lasting remission without the need of further treatment.
No significant effect of 4 infusions of bortezomib on the underlying B-cell clone was expected. However, some activity was shown by the >50% decrease of the monoclonal immunoglobulin in 23% of evaluable cases and by the reduction or disappearance of the lymphoid infiltrate in bone marrow in 2/5 patients, suggesting that a more prolonged administration may be beneficial also on the underlying B-cell disorder.
In conclusion, this study shows that a short course of bortezomib may be effective in one-third of patients with symptomatic CAD failing previous treatments, with acceptable toxicity and long-lasting benefit in the majority of responding patients. These data provide a rationale for further investigating the potential benefits of bortezomib either by using a more prolonged treatment schedule or in combination with other agents, particularly rituximab.
Acknowledgment
Janssen-Cilag Spa provided bortezomib free of charge as well as financial support to the GIMEMA Study Group for study monitoring.
Authorship
Contribution: G.R. and W.B. designed and performed research and wrote the paper; F.Z. and D.G. performed research and wrote the paper; B.F., F.B., M.D., M.F., E.L., F.R.M., F.S., and M.M. included and followed patients; F.P. and P.F. monitored and analyzed data; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: G.R. received consultant fees from Janssen-Cilag and Roche. F.Z. received fees for consultancy from Novartis, Roche, Gilead, Janssen-Cilag, Mundipharma, and Roche and received payment for lectures, including service on speakers bureaus by Roche and Janssen. W.B. received consultant fees from Bioverativ and Agios. The remaining authors declare no competing financial interests.
Correspondence: Giuseppe Rossi, ASST Spedali Civili, ple Spedali civili, 1, Brescia 25123, Italy; e-mail: giuseppe.rossi@asst-spedalicivili.it.