Abstract
Introduction: Hodgkin lymphoma (HL) is a cancer that originates in the lymphocytes. Current standard of care (SOC) therapies include combination chemotherapy and/or radiotherapy regimens. However, optimising treatment to obtain high efficacy with low toxicity remains a challenge. A systematic literature review (SLR) was conducted to identify relevant clinical evidence for front line treatments in the management of patients with advanced Hodgkin lymphoma (HL) (defined as stage IIb, III, and IV).
Methods: A SLR was performed in July 2016 to summarise the efficacy and safety of front-line treatments for the management of patients with advanced HL.
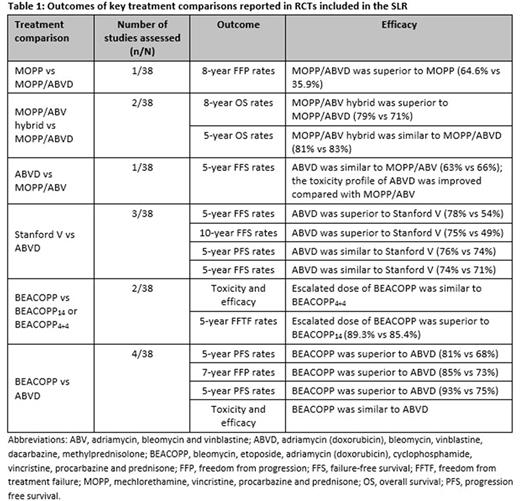
Results: In total, 70 unique studies, 38 randomised controlled trials (RCTs) and 32 non-RCTs, were identified and included in the review. Of the 38 included RCTs, nine were open-label trials, while the remaining 29 trials did not report details of blinding. Most patients with advanced HL reported durable remissions following front-line SOC treatment (ABVD and escBEACOPP) (Table 1); however, these treatment options are associated with adverse events. Further, up to 30% of advanced HL patients relapse after frontline treatment, indicating an unmet need for new therapies. The incidence of secondary malignancy at long-term follow-up after HL treatment remains a major adverse effect. Nine of the included studies reported secondary malignancy in 1.7-8.3% of patients, with acute myeloid leukaemia and non-Hodgkin lymphoma the most commonly observed secondary malignancies. However, the follow-up across these studies ranged from 5 to 8 years and the incidence of secondary malignancy are potentially underestimated due to limited follow-up. A single study reported that the cumulative incidence of secondary malignancies was 48.5% at 40 years follow-up. The risk of secondary malignancy was increased in patients receiving combined modalities compared with chemotherapy or radiotherapy alone and the risk is greater in men compared with women. The risk of second malignancy increases after initial therapy and remains elevated at ≥35 years after treatment. In the majority of studies radiotherapy was administered either as adjuvant or consolidation therapy. The most commonly reported adverse effects observed in patients receiving radiotherapy were nausea/vomiting, infections, secondary malignancy, and toxicities related to cardiovascular and pulmonary systems. Pulmonary toxicity was reported in up to 36% of patients across 23 studies with bleomycin-containing regimens. Discontinuation or dose reduction of bleomycin was associated with a decrease in the incidence of pulmonary toxicity; however, the impact of bleomycin dose reductions on efficacy outcomes was not statistically significant. Neutropenia and febrile neutropenia are also dose-limiting events that occur during chemotherapy in patients with malignant lymphoma. Granulocyte colony-stimulating factor, a haematopoietic growth factor which prevents neutropenia/febrile neutropenia, was used for primary or secondary prophylaxis in 7 and 5 of the studies included in the SLR, respectively.
Conclusion: Failure rates of up to 30% and serious adverse events are reported following treatment with current standard of care therapies in newly diagnosed patients with advanced HL. Therefore, there exists an unmet medical need for an effective and well-tolerated therapy in HL.
Dalal: Takeda Pharmaceuticals International Co: Employment, Equity Ownership. Mitchell: DRG Abacus: Employment. McCloskey: DRG Abacus: Employment. Zagadailov: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Gautam: Takeda Pharmaceuticals International Co: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.