Abstract
Background: Idelalisib (IDELA) is a potent and selective PI3Kδ inhibitor approved for the treatment of relapsed chronic lymphocytic leukemia (CLL) (in combination with rituximab) and relapsed indolent non-Hodgkin lymphoma (iNHL) subtypes, follicular lymphoma, and small-cell lymphocytic lymphoma. The relationship between plasma exposures of IDELA and clinical efficacy and safety were evaluated in relapsed/refractory CLL subjects following administration of IDELA 150 mg twice daily (BID) in combination with bendamustine (B) and rituximab (R; Study GS-US-312-0115).
Methods: IDELA peak and trough plasma samples were collected every 4 weeks (up to 24 weeks) starting at baseline. Data for safety and efficacy endpoints were collected every 2 and 12 weeks, respectively. IDELA plasma exposures (Cmax, AUCtau, and Ctau) were generated using a previously developed population pharmacokinetic (PK) model (Jin F, et al. CCP . 77: 89-98, 2016). Efficacy and safety data from the phase 3, randomized, double-blind, placebo-controlled study (GS-US-312-0115) were included in the PK/pharmacodynamics (PD) analyses. Efficacy endpoints, including best overall response rate (BOR), duration of response (DOR), progression-free survival (PFS), sum of products of the greatest perpendicular diameters (SPD) of index lesions, and lymph node response rate (LNR), were evaluated against IDELA exposures (AUCtau and Ctau). Safety endpoints, including Grade 3 or 4 adverse events of neutropenia, skin rash, infection, pneumonia, pneumonitis, diarrhea, colitis, and aspartate aminotransferase (AST) or alanine aminotransferase (ALT) elevation, were evaluated versus IDELA exposures (AUCtau and Cmax). The relationship between average daily dose of IDELA versus exposures (AUCtau and Cmax) was also evaluated. Statistical analysis (such as Kaplan-Meier survival analysis, Cox proportional hazard model, or logistic regression) was conducted for selected efficacy and safety endpoints.
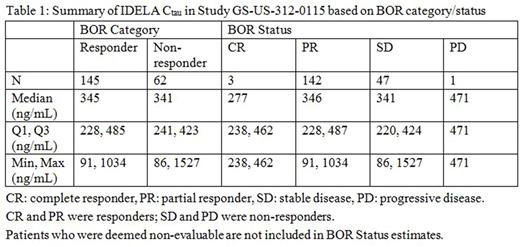
Results: Median IDELA exposures were similar in subjects who achieved an objective response (complete response or partial response) and subjects who did not respond (stable disease or progressive disease) and across the 4 response categories (p=0.68; Table 1). As IDELA exposure increased over quartiles, there was no clear trend of prolonged DOR (p=0.50), PFS (p=0.69), or change in SPD. No relationship between LNR (responder versus non-responder) and IDELA exposure was observed. No association was observed between IDELA exposure vs incidence of neutropenia (p=0.35), diarrhea (p=0.78), pneumonia (p³0.12), colitis (p=0.62), AST or ALT elevation (p=0.45), skin rash, and pneumonitis. An increase in the incidence of Grade 3 or 4 infections with higher IDELA exposures was observed (p=0.021). Overall, IDELA in combination with BR was well tolerated, as indicated by the comparable average daily doses (~300 mg) administered across quartiles of IDELA exposure.
Conclusions: No exposure-response relationships were observed for efficacy or safety (except infections) endpoints at IDELA 150 mg BID. These results are consistent with the previously observed IDELA exposure-response relationship in subjects with CLL (in combination with rituximab or ofatumumab) and iNHL. Overall, this analysis supports IDELA 150 mg BID in combination with BR for the treatment of relapsed CLL in patients who are fit for immunochemotherapy.
Daryani: Gilead Sciences, Inc.: Employment, Equity Ownership. Sharma: Gilead Sciences, Inc.: Employment, Equity Ownership. Xing: Gilead: Employment, Equity Ownership. Silverman: Gilead Sciences, Inc.: Employment, Equity Ownership. Adewoye: Gilead Sciences, Inc.: Employment, Equity Ownership. Mathias: Gilead Sciences, Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.