Abstract
Leukemia is a malignant hematological disease in which excessive clonal proliferation, deregulation, and altered differentiation of hematopoietic stem cells can occur, affecting bone marrow, peripheral blood and other tissues. Advanced age is considered an adverse prognostic factor, even after taking into account other risk factors such as cytogenetics, molecular genetics, type of acute leukemia and the patients' physical state. In elderly patients with or without treatment, the prognosis is poor, with a median estimated survival of two months and only 6% survival at two years.
In our service, elderly patients are treated with a protocol of non-intensive chemotherapy. The purpose of our protocol, called LAMMP-branch B or palliative or non-intensive treatment of acute leukemia with very poor prognosis, is to decrease hospital admission of patients and provide chemotherapy with the lowest possible toxicity. We performed an observational, longitudinal, analytical, retrospective and unicentric study from September 2005 to September 2016. The universe of this study was made up of patients over 65 years of age who were treated by the service of Adult Hematology at National Medical Center, who were diagnosed with acute leukemia and who received non-intensive chemotherapy. The primary end point was overall survival of elderly patients diagnosed with acute leukemia undergoing non-intensive chemotherapy protocol LAMMP-branch B. Secondary end points included hematological response achieved, side effects presented by patients undergoing this protocol, need for hospitalization of elderly patients in the LAMMP-branch B protocol and in which phase hospitalization occurred more frequently.
A total of 99 files were reviewed, 37 patients met the inclusion criteria. Fifty four percent (20 patients) of the patients were male, with a mean age of 72 years (64 to 87). The most frequent diagnosis was acute myeloid leukemia (AML) with 76% of cases, distributed as follows according to the FAB classification , M1 10.8%, M2 35%, M3 2.7%, M4 21.6%, M5 0%, M6 2.7% M7 2.7%. Eighty six percent (86.5%) presented some type of comorbidity, being hypertension (HTN), and the association between diabetes mellitus (DM) and (HTN), the most common with 19% respectively.
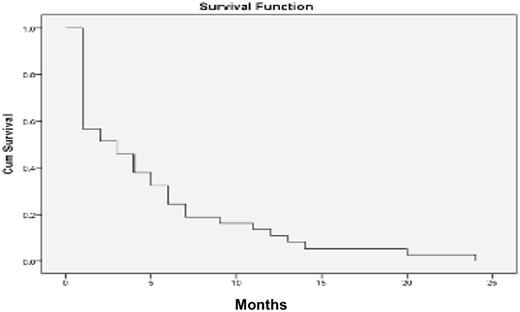
Thirty-five percent (35%) of patients treated with this LAMMP-B protocol achieved some degree of hematological response. Median overall survival was 5 months, ranging from 1 to 24 months (figure 1). During this period patients received a median of 4 cycles of chemotherapy (1 to 19 cycles), with 46% of patients presenting toxicity in 46%, of which 37.8% corresponded to toxicity grade 3-4; requiring hospital admission at least once per cycle of chemotherapy. The most frequent cause of hospital admission was need for transfusion (38%), the second most frequent cause being infections (16%). Of the 3 phases of treatment that comprise the LAMMP-B protocol, phase 1 was the one with the highest mortality rate, as well as the highest number of hospital admissions. The median days of hospitalization was 4.76 days, with a minimum of 1 day of hospitalization and a maximum of 28 days. In 2011, in our hematology service, a study was carried out in which the primary end point was to compare overall survival (OS) in patients over 60 years of age with a diagnosis of AML, treated with intensive chemotherapy (ICT) or non-intensive chemotherapy (NICT). Patients were treated during the last 11 years, concluding that the overall survival is the same with the use of ICT or NICT with less need for hospitalization in the second, with an average of 4 months. (20) Changes were made to the treatment protocol used for patients over 65 years of age, with a 1 month increase of treatment (mean of 5 months) in our study. The main objective of our current LAMMP-B protocol is to improve quality of life, in this study we observed that each patient had at least 1 hospitalization per month of survival, most with short stay for transfusions.
LAMMP-branch B,
Phase 1:
- Cytarabine 20 mg / m2 SC / day subcutaneously, days 1 to 5.
-Fludarabine, 30 mg / m2 SC, VO, days 1 to 5.
-Pegfilgrastim at day 6 continue with once a week, until recovery.
Phase 2:
- Mercaptopurine 60 mg / m2 SC / day VO, daily (use fludarabine 20 mg / m2 SC once a week) plus Metotrexate 12 mg / m2 SC / day on Tuesday and Friday, all together for four weeks.
Phase 3:
- Busulfan 15 mg / m2 / day for 4 continuous days.
- 26 days after starting again 1.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.