Abstract
Introduction
Venous thromboembolism (VTE) is a common clinical diagnosis encountered in the hospital and it is imperative to investigate the etiology of the thromboembolic event after initiating appropriate treatment. According to the American Society of Hematology Choosing Wisely Campaign, VTE provoked by a temporary risk factor does not warrant further evaluation for heritable risk factors of thrombophilia. Inappropriate thrombophilia testing is costly and may increase adverse events if anticoagulation treatment is extended based on these results. We aimed to identify the prevalence of improper thrombophilia testing in the inpatient setting and institute an EPIC advisory to prevent inappropriate use.
Methods
We conducted a retrospective chart review of patients hospitalized in our institution between January 2014 through December 2014. Adult patients (<18 yo) with admission and discharge ICD-10 codes of deep venous thrombosis and pulmonary embolism were identified. Charts were reviewed to determine etiology of VTE (i.e. unprovoked or provoked). Our primary outcome was identification of the total number of provoked VTE in the inpatient setting that underwent unnecessary thrombophilia testing. A thrombophilia evaluation was defined as any of the following tests: factor V leiden mutation, prothrombin G20210A mutation, antithrombin III activity, lupus anticoagulant, beta-2 glycoprotein 1 IgM/IgG, anticardiolipin IgM/IgG, protein C or protein S activity, MTHFR mutation, and Factor VIII activity. We then implemented an EPIC Advisory in March 2015 that appeared upon attempts to order thrombophilia tests in the inpatient setting. We allowed for a one month lead-in period. After a period of 6 months of the intervention, we performed a prospective chart review to assess for change in the total number of provoked VTEs with concomitant thrombophilia evaluations.
Results
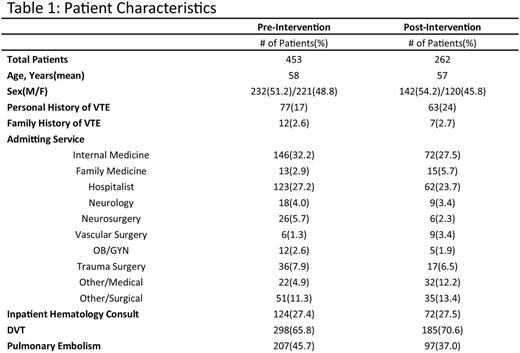
In the one year prior to the Epic Advisory, a total of 453 patients were diagnosed with provoked VTE in the hospital. Fifty-one patients (11.2%) underwent an inpatient thrombophilia work-up. Thirty-seven patients (72.5%) had at least one test ordered while on anticoagulation and twenty-three (45.1%) thrombophilia evaluations had at least one positive finding. Changes in clinical management based on thrombophilia testing were clearly documented in only 2 (0.004%) of the patients. Following the intervention, a six month time span was reviewed. We identified 262 patients with provoked VTE. Twenty patients (7.6%) underwent an inpatient thrombophilia work-up. The rates of thrombophilia testing between the pre and post intervention groups were analyzed using Fisher's exact test and results were not found to be statistically significant (P value= 0.152). Twelve (60%) of thrombophilia evaluations had at least one test ordered while on anticoagulation. Eight (40%) of thrombophilia evaluations had at least one positive finding. Changes in clinical management based on thrombophilia testing were clearly documented in two (10%) of the cases.
Discussion
In patients with provoked VTE, there is significant prevalence of inappropriate thrombophilia testing at our institution. An EPIC clinical practice advisory message incorporating the ASH Choosing Wisely recommendations trended toward limiting inappropriate testing. Review of our advisory intervention showed a modest decrease in unnecessary thrombophilia testing, but one that was not statistically significant over a six month period. This lack of significant change may be secondary to provider alert fatigue among inpatient EMR users. Providers at our institution are continually faced with EMR alerts upon placing electronic orders. Given the minimal downside associated with the advisory, we intend to continue this intervention to promote efficient use of health care resources and reduce costs associated with unwarranted testing. In the future, we could also consider inpatient ordering restrictions and further provider education given the narrow range of clinical utility for inpatient thrombophilia tests. These interventions could potentially reduce the risk of adverse events associated with extended anticoagulation and result in cost savings to the health care system.
Rajasekhar: Alexion: Consultancy; Octapharma: Consultancy; Bayer: Consultancy; Baxter: Consultancy; American Society of Hematology: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.