Abstract
Background:CPI-613 is a first in class agent that inhibits pyruvate dehydrogenase (PDH) and α-ketoglutarate dehydrogenase. We have previously shown CPI-613 inhibits mitochondrial respiration, causes phosphorylation of PDH, activation of adenosine monophosphate activated kinase in acute myeloid leukemia (AML) cells. We also showed that CPI-163 could be safely combined with high dose cytarabine (HiDAC) and mitoxantrone in patients with relapsed or refractory AML. Early efficacy data suggested that this combination increased the response rate and prolonged survival in elderly patients when compared to historical data.
Methods:We opened a Phase II trial of CPI-613 in combination with HiDAC and mitoxantrone for relapsed or refractory AML patients to confirm the safety and efficacy seen in the Phase I trial, as well as to expand several dosing cohorts. In the current trial, patients are given CPI-613 as a 2-hour infusion on days 1 through 5. HiDAC is dosed at 3,000 mg/m² (if younger than 60) or at 1,500 mg/m² (if 60 years of age or older), given every 12 hours for 5 doses, starting on day 3 following the CPI-613 infusion. The mitoxantrone was dosed at 6 mg/m² and is given once daily following the first, third and fifth HiDAC doses. At day 14, nadir marrow was evaluated and, if significant residual leukemia was present, patients could be re-treated as above or with an abbreviated 3-day cycle. Nadir marrow was re-evaluated in patients receiving a second cycle, and those with residual disease at that point were removed from study and considered refractory. Responding patients were eligible to receive up to 2 abbreviated 3-day consolidation cycles. Patients who could not go on to allogeneic stem cell transplant could receive maintenance therapy consisting of CPI-613 at 2,500 mg/m², given on days 1 through 5 every 28 days. The first 7 patients were enrolled at the maximum tolerated dose of CPI-613 2,500 mg/m2, the next 17 patients were enrolled at a dose of 2,000 mg/m2 and 6 patients have been enrolled in the current 1,500 mg/m2 cohort.
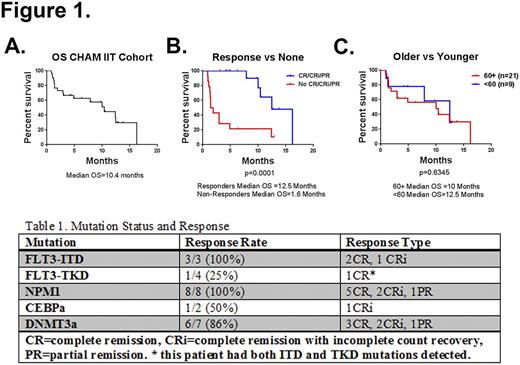
Results:At the time of this interim analysis, 30 patients are evaluable. The median age is 64 years (range 21-78). Disease was relapsed in 77% of patients (23/30) and refractory in in 23% (7/30). In patients with relapsed disease, the median duration of first complete response (CR) was 12 months. In 80% of patients, this was their first salvage regimen. AML was secondary in 5 patients, 4 with prior MDS and 1 with prior atypical chronic myeloid leukemia. Cytogenetics were poor risk in 12 patients, intermediate in 16, and good in 2. In the intention to treat (IIT) cohort (n=30) the overall response rate was 53% (10 CR + 5 CR with incomplete count recovery + 1 partial response), with a median survival of 10.4 months (Figure 1A). Response was significantly associated with survival, with a median survival of 12.5 months in responders compared to 1.6 months for all others (Figure 1B). In patients ≥60 years old, the response rate was 48% (10/21) with a median survival of 10 months. Consistent with the Phase I results, this was not significantly different from patients younger than 60 years old (p=0.6345, Figure 1C). After hematological and infectious, the most common clinically significant Grade 3 or 4 toxicities of the combination were diarrhea and anorexia. The 30-day mortality rate was 7% (2/30). Seven patients (23%) went on to allogeneic stem cell transplantation, 5 of whom were 60 years of age or older. Mutational status for FLT3, NPM, or CEBPa was available for all patients enrolled. Additional mutational testing was available for 57% of patients. In an exploratory analysis of recurring mutations, we found that 6 of 7 patients (86%) with a DNMT3a mutation achieved a response, as did all 8 patients (100%) with an NPM1 mutation (Table 1).
Conclusions: TCA cycle inhibition with CPI-613 is a promising novel approach in the treatment of AML patients, especially in the elderly and those with NPM1 and DNMT3a mutations.
Figure 1. Kaplan-Meier analyses of overall survival (OS) in: the entire IIT cohort (A), 60 years of age and older vs younger than 60 (B) and Responders vs No response (C). CHAM, combined high-dose AraC (cytarabine) and mitoxantrone; CR, complete remission; CRi, complete remission with incomplete count recovery; PR, partial remission.
Pardee: Rafael Pharmaceuticals: Employment, Research Funding. Ellis: Alexion: Speakers Bureau. Powell: Rafael Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.