Abstract
Introduction: In previously untreated patients (pts) with FL in the National Lymphocare Study who received immunochemotherapy, progression of disease (POD) in the first 24 months (POD24) predicted poor overall survival (OS) (Casulo et al. J Clin Oncol 2015). In the GALLIUM study (NCT01332968) in previously untreated FL pts, the primary endpoint of investigator (INV)-assessed progression-free survival (PFS) was significantly improved with obinutuzumab (GA101; G)-based vs rituximab (R)-based immunochemotherapy (Marcus et al. Blood 2016). This exploratory analysis aimed to assess whether POD24, and POD at time points other than 24 months, predicted OS in GALLIUM.
Methods: 1202 FL pts in GALLIUM received induction with G-chemo or R-chemo, i.e. G or R plus bendamustine, CHOP or CVP, followed in responders by maintenance with the same antibody. POD24 was defined as progressive disease (PD) or death due to PD within 24 months of randomization (noPOD24 = no progression in that time). The effect of POD24 status on OS was quantified using a Cox regression model with POD24 status as a time-varying covariate; other covariates were study treatment and age, with chemotherapy regimen and FLIPI score as stratification factors. In this model, POD24 influenced mortality rate from the time of the POD24 event onwards (initial status for all pts was no POD24, changing to POD24 at the time of a POD24 event). In addition, landmark analysis was performed to compare OS after 24 months between POD24 pts still alive at 24 months and noPOD24 pts based on a Cox regression model with the same covariates and stratification factors as listed above, but with POD24 status as a time-fixed covariate. Landmark analysis was also done to estimate 2-year OS rates in POD and noPOD groups after cut-offs at 6, 12 and 18 months, with results shown in Kaplan-Meier (KM) plots. The risk of a PFS event within 24 months of randomization was estimated and compared between treatment arms based on KM estimates. Post-progression mortality rates were estimated for all POD24 pts, POD24 pts who progressed between 0-6, 6-12, 12-18, and 18-24 months from randomization, and POD24 pts by treatment group. All GALLIUM pts were included in the age-adjusted Cox regression analysis with POD24 status as time-varying covariate. The data cut-off for the analysis was September 2016. Median follow-up was 41 months.
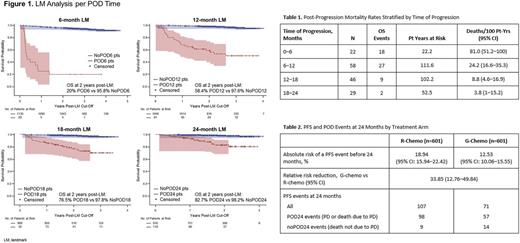
Results: At 24 months after randomization, POD24 events (PD or death due to PD) occurred in 155 pts. Compared with noPOD24 pts with at least 24 months follow-up (n=916), more POD24 pts were male (56% vs 45%), stage IV (63% vs 56%), had elevated LDH (51% vs 27%) and were high FLIPI risk (54% vs 40%). The risk of death increased 26-fold following a POD24 event (stratified hazard ratio [HR] of time-dependent POD24 status = 25.5 [95% CI: 16.2-40.3]). In a landmark analysis of 110 pts in POD24 still alive at 24 months (41 deaths and 4 censored pts excluded), the age-adjusted HR for subsequent mortality compared with noPOD24 was 12.2 (95% CI: 5.6-26.5). KM curves after the 6, 12, 18, and 24-month landmarks and post-progression mortality rates showed that mortality risk was higher the earlier pts progressed (Figure 1; Table 1). At 24 months, 18.9% (95% CI: 15.9-22.4) in the R-chemo arm and 12.5% (95% CI: 10.1-15.6) in the G-chemo arm had had PFS events, a relative risk reduction for early progression or death for any reason of 33.9% (95% CI: 12.8-49.8; Table 2). During follow-up, 56/155 POD24 pts died, 40 due to POD (15, G-chemo; 25, R-chemo) and 16 for other reasons (4 and 12, respectively), and 16/916 noPOD24 pts died, 1 due to POD (G-chemo pt who received no treatment) and 15 for other reasons (9, G-chemo; 6, R-chemo). Post-progression survival in POD24 pts was similar by treatment arm: 19/57 G-chemo pts (17.4 deaths/100 pt-years [11.1-27.3]) and 37/98 R-chemo pts (20.6 deaths/100 pt-years [14.9-28.5]) at median post-progression follow-up of ~1.85 years.
Conclusions: Analysis of POD24 in GALLIUM showed that early progression in FL pts predicts poor prognosis, in line with Casulo et al. Despite a relatively short follow-up, these data suggest that the sooner progression occurs, the greater the mortality risk, but early progressors cannot be identified in advance. G-chemo was associated with a 34% reduction in the risk of a PFS event at 24 months relative to R-chemo, including a marked reduction in the number of POD24 events. Post-progression survival for POD24 pts appeared to be similar in the two arms.
Launonen: Novartis: Equity Ownership, Other: Partner is an employee; Roche: Employment, Other: GALLIUM is sponsored by F. Hoffmann-La Roche Ltd. Third-party medical writing support, under the direction of Aino Launonen, was provided by Scott Malkin of Gardiner-Caldwell Communications, and was funded by F. Hoffmann-La Roche Ltd. Duenzinger: Roche Pharma AG: Employment. Fingerle-Rowson: F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. Nielsen: F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. Marcus: Roche: Consultancy, Honoraria, Other: Travel support, Speakers Bureau; Celgene: Other: Support for meeting attendance .
Author notes
Asterisk with author names denotes non-ASH members.