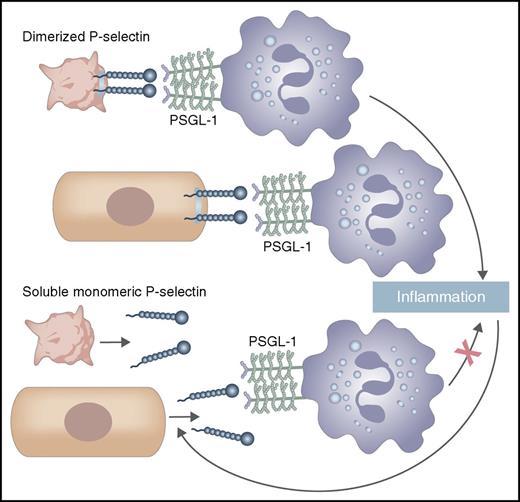
In this issue of Blood, Panicker and colleagues have clarified the role of soluble P-selectin (sP-selectin) in inflammation and cardiovascular disease by showing that monomeric sP-selectin, the form that circulates in plasma, does not promote inflammation or promote coagulation, but the dimeric form of P-selectin does.1
Dimerized P-selectin, which is expressed on the membrane of activated platelets and the endothelium, binds PSGL-1 on leukocytes to mediate adhesion and signaling, leading to inflammation. These pathways lead to proteolytic shedding of P-selectin, releasing sP-selectin, a monomer. Circulating monomeric sP-selectin binds PSGL-1 less well and does not induce leukocyte signaling, leading to inflammation.
Dimerized P-selectin, which is expressed on the membrane of activated platelets and the endothelium, binds PSGL-1 on leukocytes to mediate adhesion and signaling, leading to inflammation. These pathways lead to proteolytic shedding of P-selectin, releasing sP-selectin, a monomer. Circulating monomeric sP-selectin binds PSGL-1 less well and does not induce leukocyte signaling, leading to inflammation.
P-selectin, which is expressed on the membrane of activated platelets and the endothelium, interacts with its primary cell-adhesion partner, P-selectin glycoprotein ligand-1 (PSGL-1), on leukocytes; this occurs best when both are dimers.2 This interaction mediates leukocyte rolling and promotes inflammatory pathways. These in turn induce proteolytic shedding of the P-selectin ectodomain into the circulation, releasing sP-selectin, a monomer.2 In the circulation, P-selectin is also found on the surface of platelet-derived microparticles, where it may be a dimer, and as an alternatively spliced form. sP-selectin has been evaluated as a biomarker and is reported to be elevated in a number of conditions, including atherosclerosis, hyperlipidemia, myocardial infarction, postangioplasty stenosis,3 pulmonary arterial hypertension,4 and venous thrombosis.5,6 The prevailing view had been that sP-selectin is not only a biomarker, but directly contributes to vascular disease.7
Panicker et al show that dimerized P-selectin binds with 20-fold greater avidity than monomeric P-selectin to a glycosulfopeptide that mimics PSGL-1. Artificially, dimerization of the monomer is achieved by producing sP-selectin with an Fc tail, or by producing sP-selectin with an HPC4 tag and using an antibody directed against that tag. The authors show that sP-selectin needs to be a dimer to trigger activation of mouse leukocytes in vitro, manifest as leukocyte adhesion to ICAM-1 and to fibrinogen, and the release of citrullinated histones and neutrophil extracellular traps. After establishing the pharmacokinetics of the monomer and dimer, they demonstrate that administered sP-selectin needs to dimerize, by using the artificial means described above, to induce leukocyte rolling and firm adhesion and to increase the amount of tissue factor–bearing microparticles in blood. In a mouse inferior vena cava ligation model, administering dimeric sP-selectin increases the frequency of venous thrombosis, but administering monomeric sP-selectin does not. Similarly, administering dimeric sP-selectin shortens clotting times, but administering monomeric sP-selectin does not. Consistent with these findings, transgenic mice that overexpress monomeric sP-selectin, compared with wild-type mice, do not have altered leukocyte rolling or adherence, altered inflammatory cytokines or chemokines, or frequency of venous thrombosis. Thus, the fire is dimerized P-selectin, prevalent on membranes; (monomeric) sP-selectin is the smoke.
So how do we interpret the hundreds of publications where sP-selectin is described? Depending on assay conditions, these publications may have included microparticles in the samples where “sP-selectin” was measured. If microparticles were not specifically excluded by high-speed centrifugation, then assays for “sP-selectin” would have included microparticle P-selectin, which may be a dimer. Alternatively, perhaps microparticle P-selectin could be measured by subtracting the amount of sP-selectin in a high-speed centrifuged sample from the amount in a plasma sample.
In conclusion, although (monomeric) sP-selectin might have use as a biomarker, it is unlikely to directly contribute to disease (see figure); in contrast, agents that target dimeric P-selectin might put a brake on this coagulation-inflammation pathway.
Conflict-of-interest disclosure: The author declares no competing financial interests.