In this issue of Blood, Hunt et al elucidate the pathogenesis of a specific form of thrombotic microangiopathy (TMA) induced by the IV abuse of extended-release oxymorphone hydrochloride (Opana ER) tablets.1
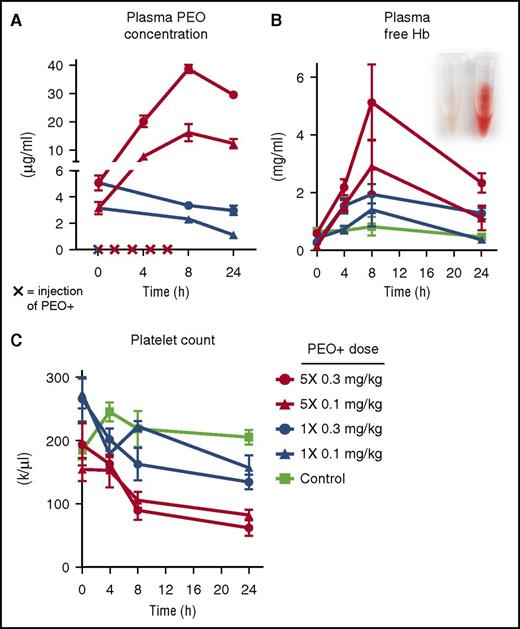
Plasma concentrations of PEO (A), free hemoglobin (Hb) (B), and platelet counts (C) in guinea pigs receiving single or repeated IV PEO+ injections (x on the x-axis in panel A). See Figure 2A-B,E in the article by Hunt et al that begins on page 896.
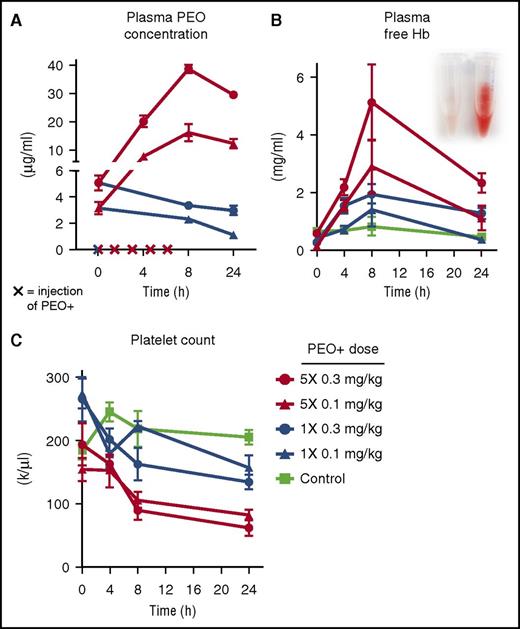
Plasma concentrations of PEO (A), free hemoglobin (Hb) (B), and platelet counts (C) in guinea pigs receiving single or repeated IV PEO+ injections (x on the x-axis in panel A). See Figure 2A-B,E in the article by Hunt et al that begins on page 896.
If a patient presents with an acute microangiopathic hemolytic anemia and thrombocytopenia with or without neurologic, renal, and/or cardiac abnormalities, most physicians rightly consider thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS) as differential diagnostic possibilities. This is justified given the need for urgent plasma exchange treatment (PEX) with fresh frozen plasma replacement and concomitant corticosteroid therapy in acquired TTP and complement inhibition by eculizumab in atypical HUS.2,3 The pathophysiology of TTP has been largely unraveled during the past 20 years: unusually large von Willebrand factor multimers, resulting from a severe acquired or hereditary deficiency of the metalloprotease ADAMTS13, lead to microvascular platelet clumping in autoimmune or constitutional TTP, respectively.4 Atypical HUS, on the other hand, has been linked to hyperactivatability of the alternative complement pathway often because of loss-of-function mutations of complement regulatory proteins, hyperfunctional mutations of complement factors C3 or factor B, and rarely to autoantibodies against factor H.2,5
Nevertheless, there are several distinct forms of TMA, clinically sometimes difficult to distinguish from TTP and atypical HUS, such as enterohemorrhagic Escherichia coli–induced typical HUS, TMA associated with hematopoietic stem cell transplantation, disseminated neoplasia, HIV infection, severe arterial hypertension, connective tissue diseases, rare metabolic disorders of cobalamin, the pregnancy complication HELLP syndrome, and certain drugs. Among the drug-associated TMAs there are toxic, dose-dependent reactions, as observed with the vascular endothelial growth factor inhibitor, bevacizumab, mitomycin C, ciclosporin, and other medications or alternatively acute immune-mediated reactions, such as seen with quinine.6,7 Finally, the first thienopyridine antiplatelet agent, ticlopidine, may lead to acquired TTP by inducing true, drug-independent autoantibodies inactivating ADAMTS13.8
In recent years, a severe acute TMA associated with the IV injection of adulterated Opana ER tablets has been observed with increasing frequency in certain areas of the United States.9 Here, Hunt et al report 3 new cases admitted to a single emergency department with an acute TMA after recent IV abuse of extracted Opana ER tablets. All 3 patients had chest pain, dyspnea, visual impairment, microangiopathic hemolytic anemia and thrombocytopenia, increased lactate dehydrogenase, and undetectable haptoglobin serum levels, and 2 had acute renal failure. PEX therapy was initiated in all 3, and additional hemodialysis was necessary in one of them. Blood samples obtained before starting PEX in 2 patients revealed normal ADAMTS13 activity, and kidney biopsies performed in 2 showed histologic evidence of TMA with endothelial swelling of arterioles and acute tubular injury without deposition of immune complexes. Remarkably, gelatinous material occluding dialysis catheter and apheresis tubings was observed during the initial PEX sessions.
The authors hypothesized that the inert ingredient mixture of Opana ER tablets, consisting mainly of high-molecular-weight polyethylene oxide (HMW PEO) of ∼7000 kD and labeled as PEO+ might be responsible for inducing acute TMA. They first simulated a typical adulteration process, heating a 40-mg Opana ER tablet cut in several pieces in a spoon with 2 mL water until boiling. With 5 consecutive extractions of a single tablet, ∼14 mg of dried PEO+ excipient was obtained. Then, using a guinea pig model, they injected animals with 0.1 or 0.3 mg/kg PEO+ IV, either as a single dose or 5× at 1.5-hour intervals. A dose-dependent increase of PEO plasma concentration peaking at 8 hours after the first dose was measured (see figure panel A), and this was paralleled by intravascular hemolysis with increased plasma concentration of free hemoglobin (see figure panel B), schistocytes in the peripheral blood smear, and thrombocytopenia (see figure panel C). No hemolysis was evidenced by spiking control blood samples with PEO+ in vitro.
In addition, after multiple IV doses of PEO+, guinea pigs showed hemoglobinuria, iron loading of the renal cortex, and histologic features of glomerular and tubular renal damage typical of TMA, as well as elevated serum creatinine and urinary albumin levels. Finally, using immunohistochemistry, HMW PEO was found in endothelium of interlobular renal arteries and capillaries and in the lumen of small renal arteries in multidosed animals. In sum, using a guinea pig model, all essential features of a TMA were evoked by (repeated) IV injections of the inert PEO+ ingredient of Opana ER tablets.
The article by Hunt et al is an excellent demonstration of translational research. Starting from the careful clinical observation of a specific drug-induced TTP-like disease, the authors proved their hypothesis of an inert ingredient-induced TMA by an appropriate animal model. Physicians involved in diagnosing and treating patients with acute microangiopathic hemolytic anemia and thrombocytopenia should consider the differential diagnosis of a TMA induced by the IV abuse of extracted Opana ER tablets.
Conflict-of-interest disclosure: B.L. holds a patent on von Willebrand factor protease (ADAMTS13), is on the Advisory Board of Ablynx for the development of caplacizumab, served as chairman of the Data Safety Monitoring Board for the BAX930 study (rADAMTS13 treatment in hereditary TTP), and received congress travel and housing support from Alexion, Baxalta, Ablynx, and Siemens.