In this issue of Blood, Bernardo et al demonstrate that monoclonal antibody combinations recapitulate the efficacy of polyclonal antibodies in suppressing red blood cell (RBC) alloimmunization. These results hold significant promise in the development of monoclonal antibody alternatives to human-derived sources for anti-Rh (RhD) immunoprophylaxis.1
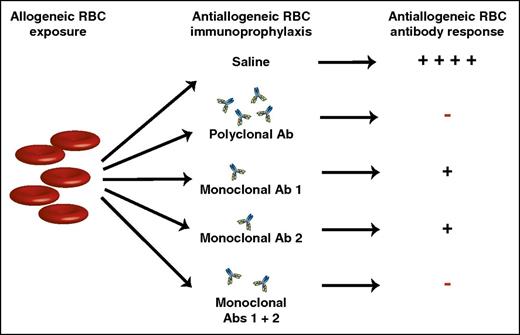
Allogeneic RBC exposure can induce alloantibodies capable of causing hemolytic transfusion reactions and hemolytic disease of the fetus and newborn. Injection of polyclonal anti-RhD antibodies can inhibit anti-RhD alloantibody formation, preventing many of the negative consequences of RhD alloimmunization. Although polyclonal anti-RhD antibodies form the basis of RhD immunoprophylaxis, previous studies have sought to identify monoclonal antibody alternatives that similarly inhibit RBC alloimmunization. Unfortunately, monoclonal anti-RBC antibodies often fail to completely prevent RBC alloantibody formation. However, by combining 2 monoclonal antibodies that target distinct alloantigen epitopes, a monoclonal antibody cocktail can be generated with the same ability to prevent RBC alloimmunization as polyclonal antibody preparations. Ab, antibody; +, antibody formation; −, no antibody formation.
Allogeneic RBC exposure can induce alloantibodies capable of causing hemolytic transfusion reactions and hemolytic disease of the fetus and newborn. Injection of polyclonal anti-RhD antibodies can inhibit anti-RhD alloantibody formation, preventing many of the negative consequences of RhD alloimmunization. Although polyclonal anti-RhD antibodies form the basis of RhD immunoprophylaxis, previous studies have sought to identify monoclonal antibody alternatives that similarly inhibit RBC alloimmunization. Unfortunately, monoclonal anti-RBC antibodies often fail to completely prevent RBC alloantibody formation. However, by combining 2 monoclonal antibodies that target distinct alloantigen epitopes, a monoclonal antibody cocktail can be generated with the same ability to prevent RBC alloimmunization as polyclonal antibody preparations. Ab, antibody; +, antibody formation; −, no antibody formation.
The discovery of ABO(H) blood group antigens resulted in the birth of transfusion medicine and allowed for the first time the use of a laboratory test that could predict immunologic outcomes prior to transfusion. Although ABO(H) antibody testing continues to represent one of the first and most common examples of personalized medicine, it was not until many years later that the existence of non-ABO(H) blood group antigens relevant in transfusion became apparent. One of the earliest descriptions of a non-ABO(H) reaction occurred following a hemolytic transfusion in the mother of a stillborn child following infusion of otherwise ABO(H)-compatible blood donated by the father.2 Additional investigation demonstrated that nearly 20% of individuals fail to express the Rh factor, a non-ABO(H) antigen also targeted on rhesus macaque RBCs following injection into rabbits.3 Ensuing studies confirmed that the presence or absence of Rh factor directly correlated with the majority of ABO(H)-compatible transfusion reactions.4
Subsequent reports demonstrated that the development of anti-Rh (RhD) antibodies might not only be responsible for ABO(H)-independent transfusion reactions, but may similarly underlie the development of hemolytic disease of the fetus and newborn (HDFN).5 Circulation of fetal RhD+ RBCs released during pregnancy or parturition appeared to stimulate anti-RhD antibody formation,6 putting subsequent RhD+ pregnancies at risk for developing HDFN. Although these studies provided key insight into factors that appeared to influence RBC alloimmunization during pregnancy, subsequent studies provided a rationale behind strategies designed to prevent RBC alloimmunization during pregnancy and thus HDFN.7 Although pregnancy with a RhD+ fetus clearly put RhD− mothers at risk for developing anti-RhD alloantibodies, ABO(H) incompatibility between a mother and fetus independently reduced the likelihood of anti-RhD antibody formation.7 The ability of anti-A and/or anti-B antibodies to inhibit anti-RhD antibody development appeared to be consistent with an observation by Theobald Smith many years earlier that administration of passive antibody can prevent immunization following exposure to a target antigen.8 These combined results suggested that naturally occurring anti-ABO(H) antibodies likely engage RhD+ fetal RBCs and prevent RhD alloimmunization.
As the conception of human life is rarely predicated on a partner’s blood group status, avoidance of RhD exposure, as routinely occurs in the setting of RBC transfusion, was not a practical intervention to prevent Rh alloimmunization during pregnancy. Given the ability of anti-ABO(H) antibodies, which are predominantly immunoglobulin M (IgM), to inhibit RhD alloimmunization, early efforts sought to generate IgM anti-D antibodies to intentionally prevent RhD alloimmunization in RhD− pregnant females. However, unlike anti-ABO(H) antibodies, IgM anti-RhD failed to similarly inhibit RhD alloantibody formation.9 As a result, subsequent studies used polyclonal IgG anti-RhD antibodies primarily isolated from women previously sensitized through pregnancy. Injection of polyclonal IgG anti-RhD significantly inhibited anti-RhD alloantibody formation in RhD− males and likewise reduced RhD alloimmunization during pregnancy. In pregnancy alone, anti-RhD immunoprophylaxis reduced the likelihood of RhD alloimmunization nearly 50-fold, all but eliminating HDFN secondary to RhD alloimmunization.9 For >20 years, these studies exemplified the power of a multidisciplinary approach when seeking to not only understand but also develop a strategy to prevent a devastating disease.
Although anti-RhD immunoprophylaxis continues to enjoy considerable success, several key drawbacks still exist. As the efficacy of RhD immunoprophylaxis reduced the incidence of RhD alloimmunization, the natural source of anti-RhD generated during pregnancy likewise decreased. As a result, manufacturing centers enrolled RhD-negative men and intentionally immunized these individuals with RhD+ RBCs to generate polyclonal anti-RhD. Although this approach is acceptable, it is prone to the inherent biological variability observed when using different human donors and continuously poses a risk of limitations in supply.10 To overcome challenges associated with polyclonal preparations, many attempts have been made to develop monoclonal anti-RhD replacements that display the same efficacy as polyclonal anti-RhD with mixed success.10 Challenges associated with developing equally effective surrogate products in part reflect a complete lack of understanding regarding the mechanism(s) responsible for anti-RhD antibody-mediated immunosuppression.
Bernardo et al demonstrate that, although individual monoclonal antibodies do suppress alloantibody formation following RBC exposure, combining 2 monoclonal antibodies that recognize distinct RBC alloantigen epitopes results in a level of suppression similar to that observed following injection of polyclonal preparations (see figure). These studies hold significant promise, as these findings suggest that blends of different monoclonal antibodies may provide a satisfactory substitute for the use of polyclonal preparations in the future. Equally important, although anti-RhD antibodies currently prevent antibody formation against RhD antigens, similar approaches do not exist for other RBC alloantigens. Indeed, in the wake of RhD immunoprophylaxis, anti-KEL antibodies are now responsible for more HDFN-related mortality than any other anti-RBC alloantibody. As a result, the present study not only holds promise in the potential development of monoclonal combinations that may replace naturally derived sources for anti-RhD, but may also serve as a platform to provide similar approaches to prevent equally devastating consequences of alloimmunization against other RBC alloantigens. As new models and mechanisms of antibody-mediated immunosuppresion become available, studies like these will continue to build on the successful history of RhD immunoprophylaxis to generate better products and thus extend this therapy to patients who continue to suffer from HDFN.
Conflict-of-interest disclosure: The author declares no competing financial interests.