In this issue of Blood, Antonelli et al1 developed a novel xenograft mouse model that mimics a humanized bone marrow (BM) niche that improved human leukemia engraftment and promoted growth and clonal diversity. This new approach is the next step in decades of work to recapitulate leukemia development and growth in mice: from initial studies using syngeneic murine leukemia cell lines; then, engineering transgenic mice or hematopoietic stem cells for expression of leukemia oncogenes; and, more recently, using a variety of permissive immune-deficient mouse strains that support robust xenografting with primary human leukemia samples.
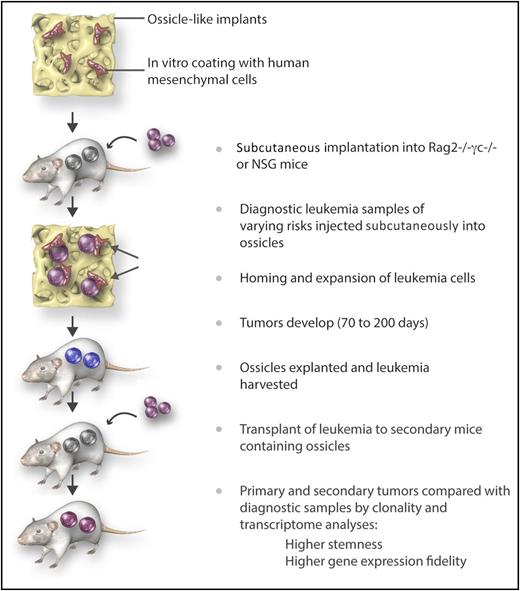
Steps for improved outgrowth and maintenance of the genetic signature of human diagnostic leukemia samples in humanized “ossicles,” which mimic the natural niche in vivo. Immune-deficient mice were implanted subcutaneously with calcified scaffolds previously seeded with human MSCs. Six to 8 weeks later, diagnostic leukemia cells (CD34+ purified or T cell–depleted) were injected. Tumors were explanted and leukemia cells transferred to ossicles in secondary recipients. This technique was shown to result in a better engraftment rate of leukemia samples with favorable risks than the traditionally used IV administration technique. In addition, the self-renewal and genetic profile of the original leukemia was better maintained. Professional illustration by Somersault18:24.
Steps for improved outgrowth and maintenance of the genetic signature of human diagnostic leukemia samples in humanized “ossicles,” which mimic the natural niche in vivo. Immune-deficient mice were implanted subcutaneously with calcified scaffolds previously seeded with human MSCs. Six to 8 weeks later, diagnostic leukemia cells (CD34+ purified or T cell–depleted) were injected. Tumors were explanted and leukemia cells transferred to ossicles in secondary recipients. This technique was shown to result in a better engraftment rate of leukemia samples with favorable risks than the traditionally used IV administration technique. In addition, the self-renewal and genetic profile of the original leukemia was better maintained. Professional illustration by Somersault18:24.
The novelty in the current report is the human-like BM niche, which consisted of calcified scaffolds containing human mesenchymal stromal cells (MSCs) that were implanted into immune-deficient (NOD/SCID IL2Rγc−/− or RAG2−/− IL2Rγc−/−) mice (see figure). Analyses of these implants after several weeks demonstrated formation of bones and blood vessels resembling ossicles. Four to 6 of these humanized BM scaffolds (huBM-sc) were implanted subcutaneously in each mouse, and 6 to 8 weeks later, diagnostic leukemia cells from untreated patients (CD34+ purified or T cell–depleted) were injected. Thirty-nine patient samples with favorable (36%), intermediate (21%), and adverse (44%) risk acute myeloid leukemia (AML) were tested for outgrowth of leukemia-derived tumors in the ossicles and in the mouse BM. For comparison, murine BM-iv (muBM-iv; ie, mice given IV leukemia samples that would only home in the mouse BM) were run in parallel. Engraftment and outgrowth of leukemia cells was seen in 3 of 7 cases with the muBM-iv model (43%, determined as 30% to 60% huCD45+ cells in peripheral blood and signs of illness) vs 29 of 39 cases in the huBM-sc model (74%, determined as the biggest tumor growing up to 1.5 to 2.0 cm3, at which point the mice had to be euthanized).
The first important observation of this large study was that leukemia samples with favorable risk, which are notoriously hard to grow in immune-deficient mice, grew consistently in the huBM-sc model, but not at all in the muBM-iv model. The authors further convincingly demonstrated that engraftment of leukemia in the huBM-sc model was also qualitatively more informative than in the muBM-iv model: leukemic cells isolated from the humanized scaffolds showed superior secondary transplantation capacity compared with leukemic cells isolated from the murine BM niche. Importantly, even a favorable risk leukemia sample could be transplanted from primary to secondary mouse recipients. This supported the hypothesis that leukemia stemness could be better preserved in a species-specific BM niche.
Further, clonal heterogeneity and clonal drift were evaluated by variant allelic frequencies (analyzed by exome sequencing), and showed substantially higher clonal diversity in the huBM-sc model than in the muBM-iv model. Finally, transcriptome studies were performed to compare samples of leukemia grown in the huBM-sc and in the muBM-iv models. Supervised clustering within individual patient samples showed that the leukemia signature was more conserved for the huBM-sc model. The Schuringa team has previously reported equivalent findings using the same scaffold/allogeneic human MSC implants, but making use of human cord blood CD34+ cells genetically modified to express leukemia oncogenes (BCR-ABL and MLL-AF9).2 Higher frequencies of leukemia development and more consistent stemness were observed in the huBM-sc than in the muBM-iv model. In a recent study published by Reinisch et al,3 a similar reconstructed niche was described that consisted of BM-derived MSCs admixed with an extracellular matrix (Matrigel) implanted subcutaneously in the NSG mice. In this case, daily injections for 4 weeks with an anabolic dose of human parathyroid hormone resulted in endochondral ossification and marrow cavities repopulated with hematopoietic cells. Injection of primary leukemia cells (7 AML, 4 acute promyelocytic leukemia, and 2 biphenotypic leukemia) into these ossicles resulted in accelerated engraftment with substantially higher frequencies of leukemia-initiating cells. Further supporting the findings from the Schuringa laboratory, Reinisch et al also showed that AML cells engrafted in humanized ossicles recapitulated the original clonal diversity with more fidelity than mice injected with leukemia IV.3
In still another experimentally more complex setting, Medyouf et al reported on primary Lin−CD34+CD38+ cells from samples from patients with myelodysplastic syndromes matched with the autologous MSCs in orthotopic NSG xenografts.4 Here as well, the malignant clonal diversity was more effectively recapitulated in the presence of MSC support. Notably, however, if heathy MSCs were used, the myelodysplastic syndrome samples were able to subvert the allogeneic niche into producing abnormal niche factors. Altogether, these different complementary publications indicate that human MSCs instruct and are instructed by leukemia. The relevance of these works is that they set a new benchmark for xenograft models of human leukemogenesis beyond just the use of immune-deficient strains that express several combinations of human cytokines that can potentially activate myeloid cells, including leukemia cells.5
Eventually, the incremental combination of new engineered mouse strains with innovative ossicle implants will indeed allow a deeper understanding of the niche and spatiotemporal events steering the human polyclonal or oligoclonal leukemogenesis. In addition, because leukemia cells also interact with and ultimately subdue the immune system, the next exciting frontier will be to transfer these sophisticated leukemia engraftment models into fully humanized mouse systems containing functional human adaptive immune responses.6 For this, 1 strategy would be to coadminister long-lasting stem cell–matched dendritic cells to generate mature human T and B cells and adaptive responses in fully humanized mice.7 Once all of these key components are combined, advanced human-specific immune therapies such as check-point inhibitors8 and engineered T cells expressing chimeric antigen receptors9 could be tested for proof-of-concept and efficacy in fully humanized mouse models containing primary leukemia + niche + immune system before entering highly demanding and costly clinical trials.
Conflict-of-interest disclosure: The author declares no competing financial interests.