Abstract
Introduction. Upper extremity deep vein thrombosis (UEDVT) is a relatively uncommon event with potentially serious complications. Its clinical outcomes are not well studied. The objective of this study was to assess the incidence of post-thrombotic syndrome (PTS) and functional disability in patients with UEDVT.
Patients and methods. This was a pre-specified analysis of a prospective cohort study at 5 Canadian centres. We enrolled adult patients with a symptomatic UEDVT confirmed by compression ultrasound involving the brachial or more proximal veins, with or without a pulmonary embolism (PE). Exclusions included pregnancy, dialysis catheter thrombosis, active or high bleeding risk, platelet count <100x109/L, creatinine clearance < 30 ml/min, on warfarin for other indications, hemodynamically unstable PE, acute leukemia or undergoing a stem cell transplant within 3 months, geographical inaccessibility, life expectancy <3 months or treatment with low molecular weight heparin (LMWH) or warfarin for more than 7 days since diagnosis. Standardized treatment regimens were used as follows: spontaneous or central venous catheter (CVC)-related UEDVT were treated with dalteparin at therapeutic doses for at least 5 days followed by warfarin adjusted according to INR results. Spontaneous UEDVT was treated for at least 6 months and CVC-related events were treated for at least 3 months or for as long as the line remained in place and for at least 1 month after line removal. Cancer patients with non CVC-related UEDVT were treated using dalteparin alone for a minimum of 6 months. Outcomes were assessed at 12, 18 and 24 months and included the occurrence of PTS evaluated using a modified Villalta Score. PTS was defined for patients with a score of 5 or greater and severe PTS was defined by a score of 15 or higher. Functional disability was evaluated using the Disabilities of the Arm, Shoulder and Hand (DASH) questionnaire. Patients were followed for 2 years. Data was analyzed using descriptive statistics. Confidence intervals for proportions were estimated using the Wilson's score method. Groups were compared using χ2 and Student's t- tests, as appropriate. The study was approved by all institutional review boards.
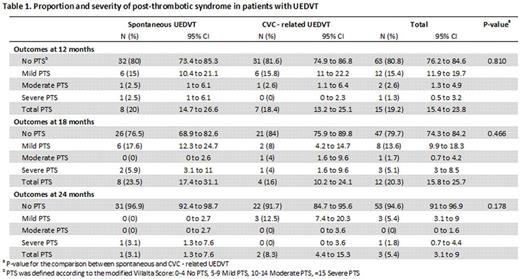
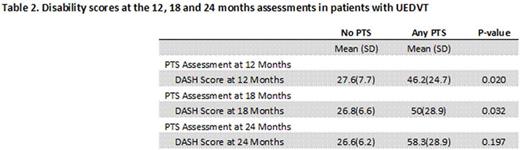
Results. Between 2009 and 2012, we enrolled 141 patients: 75 with spontaneous and 66 with CVC related UEDVT. Mean age was 51 years and 55% were males. There were 78, 59 and 56 patients with evaluable data at 12, 18 and 24 months, respectively. The percentage of patients with ipsilateral PTS is shown in Table 1. There was no difference between patients with spontaneous or CVC- related UEDVT. Functional disability scores are shown in Table 2. Overall, patients developing PTS had higher functional disability at all time points, compared to patients without PTS.
Conclusion. In this prospective cohort study PTS occurred in approximately one fifth of patients after UEDVT and was associated with more functional disability, although the majority of cases were mild according to the modified Villata score. No differences were observed between CVC-related and spontaneous UEDVT.
Lazo-Langner:Bayer: Honoraria; Pfizer: Honoraria; Daiichi Sankyo: Research Funding. Wells:Itreas: Other: Served on a Writing Committee; BMS/Pfizer: Research Funding; Bayer Healthcare: Other: Speaker Fees and Advisory Board; Janssen Pharmaceuticals: Consultancy. Carrier:BMS: Research Funding; Leo Pharma: Research Funding. Kovacs:Daiichi Sankyo Pharma: Research Funding; LEO Pharma: Honoraria; Bayer: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.