Abstract
The incidence of inherited rare bleeding, thrombotic and platelet disorders (BPD) is estimated to be 200-250 per million individuals. A considerable portion have heritable thrombocytopenia (HT), a genetically heterogeneous group of disorders with which 27 of the 76 ISTH-approved Tier 1 BPD genes have been causally associated. It is important to distinguish cases because variants in some HT genes confer risk of malignancy and pathologies outside hemopoiesis. This can be seldom achieved by considering clinical and laboratory characteristics alone. In order to improve the precision of diagnosis for HT subtypes and to identify novel genes underlying HT, we have developed two complementary high throughput sequencing (HTS) approaches. The first is for BPD cases with an assumed molecular diagnosis; 500 DNA samples have been sequenced with the ThromboGenomics HTS gene panel test for the 76 Tier 1 BPD genes. The second is for patients with rare disorders of unclear molecular aetiology enrolled in the NIHR BioResource. To identify the genetic basis of these rare disorders 10,000 DNA samples have been whole genome sequenced, including samples from 1,378 BPD probands, 123 affected and 41 unaffected relatives. Upon consenting, clinical parameters, laboratory results and pedigree history were deposited in a shared single study database and human phenotype ontology (HPO) terms appended to each patient. Novel statistical methods have been deployed to identify causal variants. Here we report on the pathogenic and likely pathogenic variants (PV and LPV) in 27 Tier 1 genes identified in cases with HT who had completed evaluation by either approach by July 2016.
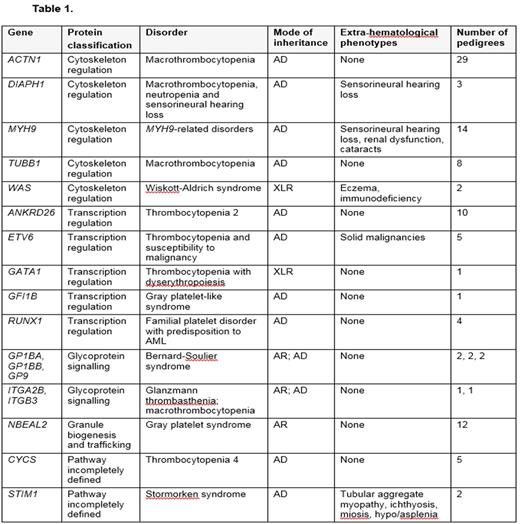
We defined PV as either high impact variants or variants present in 4 or more independent HT cases reported in the literature or our study database, and LPV as other credible variants with fewer than 4 independent cases previously observed. All cases were reviewed at a multi-disciplinary team meeting before being reported as "explained", "unexplained" or "partially explained" if components of the broader phenotype could not be explained by the variants observed. Between January 2011 and July 2016, 119 cases from 104 pedigrees with HT completed analysis with the identification of a PV or LPV in a Tier 1 HT gene believed to explain or partially explain the described phenotype. Variants were identified in 18 of the 27 HT genes (Table 1), including genes encoding transcription regulators (11/104 pedigrees) and platelet membrane proteins (8/104 pedigrees). However, variants in 5 genes encoding cytoskeletal components or regulators comprised the largest single functional group (57/104 pedigrees).
We identified 18 different PV or LPV (8 novel) in ACTN1 in 29 pedigrees with non-syndromic macrothrombocytopenia (MTP) and 8 different PV or LPV (6 novel) in TUBB1 in 8 further pedigrees with non-syndromic MTP. PV or LPV in Tier 1 cytoskeletal HT genes associated with thrombocytopenia and variable non-haematological syndromic features were identified in 20 pedigrees, including 3 pedigrees with 2 different gain-of-function variants in DIAPH1 causing MTP, intermittent neutropenia and sensorineural hearing impairment. One variant has recently been reported by our Consortium, the other is novel. The remainder comprised 2 PV in WAS in 2 pedigrees and 14 different PV or LPV (4 novel) in MYH9 in 15 pedigrees, some of whom had isolated MTP without neutrophil inclusion bodies. Of note, common variants in four of these five genes have been found to be associated with the count and volume of platelets in genome wide association studies, confirming the notion that platelet-GWAS loci are enriched for genes underlying Mendelian platelet disorders.
In conclusion, HT are genetically diverse disorders for which there is an unmet need for precision diagnosis. For HT cases with clinical and laboratory characteristics similar to previously reported ones for HT, the ThromboGenomics test is a valuable and affordable approach to obtain an accredited genetic diagnosis. Whole genome sequencing is a powerful approach to identify novel genes implicated in HT for the rapid enhancement of the ThromboGenomics HTS panel test. The high prevalence of variants causal of HT in Tier 1 BPD genes that encode cytoskeletal elements highlights the pivotal role of the cytoskeleton in platelet formation by mediating pro-platelet protrusion and budding from megakaryocytes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.