Abstract
Background
Salvage chemotherapy using high-dose etoposide and cyclophosphamide (VP-Cy) was developed for acute leukemia and myeloid malignancies resistant to cytarabine-based induction regimens. The Leukemia/Bone Marrow Transplant (L/BMT) program of British Columbia has a 25-year experience using VP-Cy for patients with relapsed and refractory (R/R) acute myeloid leukemia (AML). We report on a retrospective analysis to evaluate the efficacy and early toxicity of this regimen.
Methods
The L/BMT database was searched to identify R/R AML patients treated with VP-Cy. Patients were treated with etoposide 2.4 g/m2 given as continuous infusion over 34 hours on days 1-2 followed by cyclophosphamide 2 g/m2/day on days 3-5. The primary aims of this analysis were to determine response rate, long-term survival outcomes, and early toxicity. Secondary aims were to determine the impact of initial induction chemotherapy, using either standard-dose cytarabine and daunorubicin (7+3) or high-dose cytarabine and daunorubicin (HiDAC-D), on response rate to salvage as well as to assess the impact of cytogenetics and FLT3-ITD mutations on outcome. Survival curves were calculated using the Kaplan-Meier method, and comparisons between curves were made using the log-rank test. Chi-squared test was used to compare differences in categorical variables.
Results
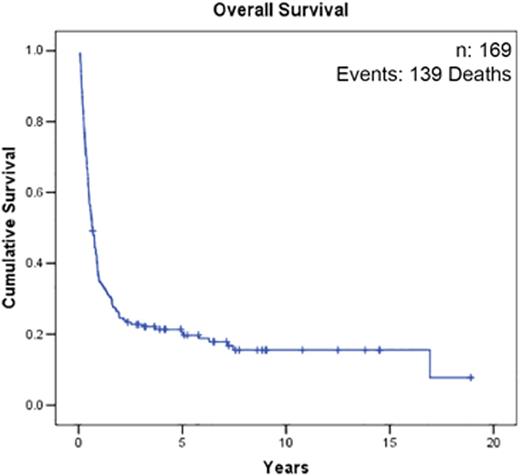
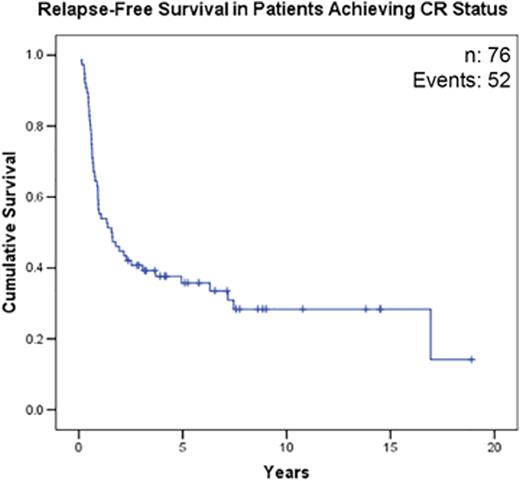
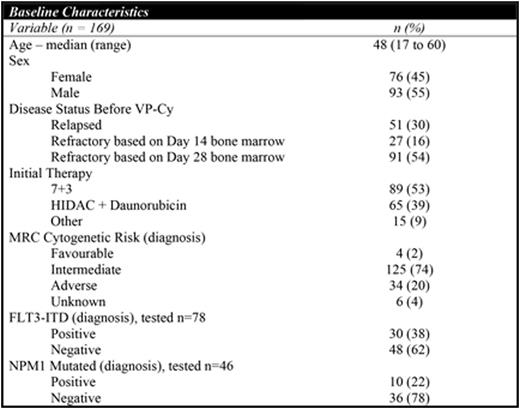
169 patients diagnosed with R/R AML treated with VP-Cy from 1991-2015 were identified. Baseline characteristics are shown in Table 1. CR rate was 45% and 5-year OS was 20% (95% CI 14-26%) for the entire cohort. Rate of CR (p=0.98) and median OS (p=0.8) were not different between cohorts receiving standard-dose cytarabine vs. high-dose cytarabine during initial induction. 74% (56/76) of responding patients received allogeneic hematopoietic stem cell transplant. 42% (32/76) of patients relapsed after initially responding to VP-Cy and the median CR duration was 221 days (range: 36 to 2561 days). In responding patients (n=76), 5-year relapse-free survival (RFS) and OS were 33% (95% CI 24-46%) and 35% (95% CI 25-47%) respectively. Initial disease status did not significantly influence response rate [52% (refractory day 14) vs. 42% (refractory day 28) vs. 47% (relapsed), p=0.46] although median OS was longer in patients with refractory disease [422 days (refractory day 14) vs. 281 days (refractory day 28) vs. 122 days (relapsed), p=0.03)]. Patients with FLT3-ITD mutation (30/78) had lower CR rate (23% vs. 53%; p=0.01) and worse OS (median OS 135 days vs. 357 days; p<0.0001) compared to those without. Patients with adverse risk cytogenetics (34/169) had a trend towards lower CR rate (38% vs. 50%; p=0.2) and a significantly worse OS (median OS 155 days vs. 304 days; p<0.0001) compared to patients with favourable/intermediate cytogenetics. Rate of early death (<60 days) was 12% (20/171) and causes included: bacterial infection (n=4), fungal infection (n=3), intracranial hemorrhage (n=1), and progressive AML (n=9).
Conclusion
VP-Cy is an effective salvage regimen with response rates, long-term survival and rates of early death comparable to reported outcomes using cytarabine-based salvage regimens. Initial dose of cytarabine given during induction did not influence response rate or survival. The probability of long-term survival for the entire cohort was low (20%) and outcomes were significantly worse in patients with FLT3-ITD mutation and adverse risk cytogenetics, emphasizing the need for investigation of novel treatments in these groups.
(a) Overall Survival. (b) Relapse-Free Survival in Patients Achieving CR Status After VP-Cy.
(a) Overall Survival. (b) Relapse-Free Survival in Patients Achieving CR Status After VP-Cy.
Song:Celgene: Honoraria, Research Funding; Janssen: Honoraria; Otsuka: Honoraria. Toze:Roche Canada: Research Funding. Gerrie:Roche Canada: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.