Abstract
Background. The TWiTCH multicentre randomised phase III trial showed recently non inferiority of hydroxyurea (HU) with phlebotomy versus transfusions with chelation for primary stroke prevention and management of iron overload in children with sickle cell anemia, having had abnormal transcranial Doppler but no severe vasculopathy and after at least one year of transfusion. Importantly, children in the HU arm received the drug at the maximum tolerated dose (MTD) with escalation of dosage until absolute neutrophil count (ANC) is <3.0 x109/L. Median HU MTD was 27.4 mg/kg/d [IQR 24.0-30.1]). Final average HbF% and ANC were 24.4% and 3.6 x109/L respectively. Most European centers do not try to reach the MTD. Our study aimed to determine median HbF% and ANC (a count <3.0 x109/L being considered as reflecting the achievement of the MTD) in the children included in the European ESCORT-HU cohort to see whether they were within the ranges demonstrated to exert brain protection in the TWiTCH trial.
Methods. We extracted data from the ESCORT-HU cohort, which is non-interventional, prospective, observational open-label study on the efficacy and safety of Siklos. The cut-off date was set as February 5th, 2016. Out of the global cohort of 992 patients, 386 patients were aged 2 to 18 years at the time of study initiation. Among them, 233 patients were HU treatment-naïve and initiated Siklos® treatment. A follow-up of at least 12 months and data allowing biological or clinical efficacy evaluations were available in a subgroup of 139 "naïve" (= not previously treated with HU) patients. Our objectives were 1) to collect the dosages of HU and to compare HbF% and ANC before and 12 months after initiation of Siklos® in the subgroup of naïve patients (table 1); 2) to assess the efficacy of HU in this cohort. We compared using Mac-Nemar paired t-test and Wilcoxon sign rank-test in the 12 months period before treatment and 12 months after initiation of treatment i) painful crises >48 hours, ii) acute chest syndromes (ACS), iii) hospitalizations, and iv) blood transfusion (% of patients having had at least 1 event and the total numbers of events); 3) to assess in the total cohort of 386 children the % of children with ANC count within the ranges recommended in the TWiTCH trial, underlining those treated with HU for cerebral vasculopathy (table 2).
Results
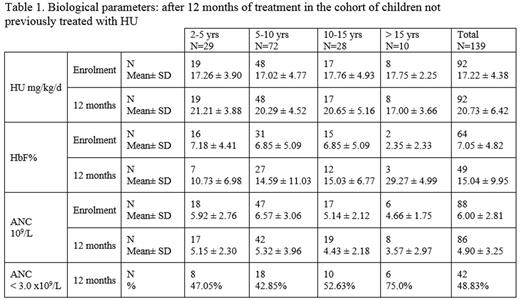
1) HbF% and ANC in the subgroup of 139 children not previously treated with HU (table 1)
46.8% were males; mean age was 8.1 ± 3.9 years. Genotypes were HbSS, HbSC, HbSb0thalassemia, HbSb+ thalassemia, and unknown in respectively 86.5%, 1.4%, 7.8%, 2.1% and 2.1% of cases.
2) Efficacy data in this subgroup
The % of patients having had at least 1 episode and the total numbers of painful crises > 48 hours, ACS, hospitalizations, and blood transfusion in the 12 months, decreased significantly under HU (P<0.001).
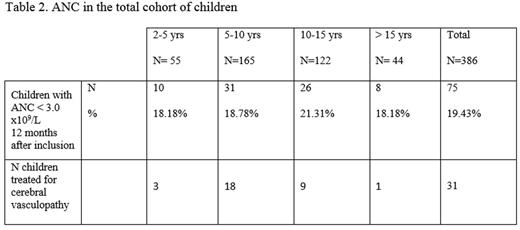
3) ANC in the total cohort of children from ESCORT-HU cohort (table 2)
The total cohort enrolled 386 children, 51.1 % were males, and mean age was 9.65 ± 4.26 years. Genotypes were HbSS, HbSC, HbSb0thalassemia, HbSb+ thalassemia, and unknown in respectively 89.1%, 2%, 4.7%, 2%, and 2.2% of cases.
Only 159 patients had one HbF% determination 12 months after inclusion. Out of these, 138 (86.79%) had ANC > 3.0 x109/L, their mean HbF was 13.04 ± 8.6 %; 21 (13.20%) had ANC < 3.0 x109/L, their mean HbF was 21.02 ± 9.64 %.
In conclusion
We show that children included in the ESCORT-cohort received median HU dosage quite lower than the one given to children included in the TWiTCH trial which resulted in quite lower HbF %. This dosage was sufficient to very dramatically reduce SCD-related painful events. Whether it can exert the same brain protection as the one demonstrated in the TWiTCH study is so far not known. We encourage physicians to reach MTD when the indication for HU is brain protection.
De Montalembert:Addmedica: Research Funding; Novartis: Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.