Abstract
Background: The International Staging System (ISS) for multiple myeloma (MM) is a simple risk stratification algorithm based on serum β2-microglobulin and serum albumin that identifies three patient groups with different prognoses. Recently, a revised ISS (R-ISS) has been created that combines ISS stage with serum lactate dehydrogenase (LDH) and chromosomal abnormalities (CAs) identified by fluorescence in situ hybridization (FISH); it has been shown to better stratify patients based on prognosis (Palumbo, et al, J Clin Oncol, 2015). With the increasing availability of deeper sequencing techniques, we sought to determine if the use of next generation sequencing (NGS) instead of FISH for the identification of CAs would further improve the stratification of higher-risk patients.
Methods: Data was extracted from the open-access MMRF Researcher Gateway corresponding with interim analysis 8 from the CoMMpass study. The CoMMpass study is enrolling 1000 newly diagnosed MM patients who will be tracked longitudinally for 5 years. CoMMpass collects relevant clinical data as well as sequential tissue samples. Eligibility requirements for CoMMpass include: symptomatic MM with measureable disease by SPEP (≥1.0g/dL), UPEP (≥200mg/24 hours), or SFLC (≥10mg/dL); receiving an immunomodulator (IMID) and/or a proteasome inhibitor (PI) for initial MM treatment; and no prior malignancies in the past 5 years. All sequencing was performed by the Translational Genomics Research Institute (TGEN). CAs were identified using custom software on long-insert whole genome sequencing data.
ISS stage was calculated used β2-microglobulin and serum albumin as previously described (Greipp, et al, J Clin Oncol, 2005). R-ISS stage was then calculated as: stage I (ISS stage I, no high-risk CA, and normal LDH); stage III (ISS stage III with high-risk CAs and/or high LDH levels); stage II others. High risk CA were defined as the presence of Del(17p), t(4;14) or (4;16). High LDH was defined as LDH ≥300units/L.
We performed a multivariate Cox regression analysis to compare event-free survival (EFS) defined as the interval from diagnosis to disease progression or death controlling for age (>65 vs ≤65 years) and sex for ISS, R-ISS (using FISH data), and R-ISS using NGS data (R-ISS-NGS). The level of significance was set at 0.05 for all tests.
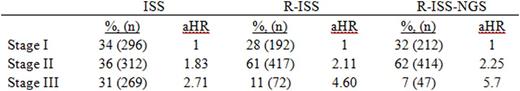
Results:877 patients had β2-microglobulin and serum albumin available and were eligible for analysis. The median age at diagnosis was 64 years, 61% (533) were male, and 76% (666) were white. 34% (296) were ISS stage I, 36% (312) were stage II, and 31% (269) were stage 3. R-ISS was calculated on the 681 patients with available FISH and LDH data. 28% (192) were R-ISS stage I, 61% (417) were stage II, and 11% (72) were stage III. R-ISS-NGS was calculated on the 673 patients with available NGS and LDH data. 32% (212) were R-ISS-NGS stage I, 62% (414) were stage II, and 7% (47) were stage III.
ISS stage was significantly associated with EFS; patients with stage II and stage III disease had an 83% (aHR 1.83 [95% CI 1.25-2.69], p = 0.0183) and 171% (aHR 2.71 [95% CI 1.85-3.95], p < 0.0001) increased risk of disease progression or death, respectively, compared to stage I patients. Using R-ISS, the model was able to better stratify patients by risk; patients with stage II and stage III disease had a 111% (aHR 2.11 [95% CI 1.32-3.37], p = 0.0017) and 360% (aHR 4.60 [95% CI 2.58-8.17], p < 0.0001) increased risk of disease progression or death, respectively, compared to stage I patients. Using, R-ISS-NGS the model was able to better stratify patients by risk particularly among stage III patients; patients with stage II and stage III disease had a 125% (aHR 2.25 [95% CI 1.42-3.55], p = 0.0005) and 470% (aHR 5.70 [95% CI 3.12-10.40], p < 0.0001) increased risk of disease progression or death, respectively, compared to stage I patients. Results are summarized in Table 1.
Conclusion: R-ISS-NGS improved on FISH based R-ISS stratification, presumably due to more accurate determination of the high-risk CAs of interest. A more thorough analysis including additional candidate CAs may further increase the sensitivity of this NGS based staging system.
Vij:Takeda: Consultancy, Research Funding; Celgene: Consultancy; Bristol-Myers Squibb: Consultancy; Janssen: Consultancy; Novartis: Consultancy; Karyopharma: Consultancy; Amgen: Consultancy, Research Funding; Jazz: Consultancy; Shire: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.