Abstract
Background:
Chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) occurs primarily in older patients (pts) who often have increased comorbidities and cannot tolerate aggressive treatments, which leads to poorer outcomes (Balducci, Cancer Control 2015; Thurmes, Leuk Lymphoma 2008). Alkylating agents, such as chlorambucil (clb), have been commonly used to treat these pts (Eichhorst, Blood 2009). Ibrutinib (ibr), a first-in-class, once-daily, inhibitor of Bruton's tyrosine kinase, is indicated by the US FDA for the treatment of pts with CLL/SLL and allows for treatment without chemotherapy. RESONATE-2 (PCYC-1115) is a randomized phase 3 trial designed to compare the efficacy and safety of ibr vs clb in pts with treatment-naïve (TN) CLL/SLL (Tedeschi, ASH 2015). Primary results, as assessed by an independent review committee (IRC), demonstrated with a median follow-up of 18.4 mo that ibr significantly reduced the risk of progression or death by 84% (P<0.001)(Burger, N Engl J Med 2015). Based on these results, ibr was approved as a first-line treatment option for CLL pts. This extension study provides updated efficacy and safety results of ibr for RESONATE-2.
Methods:
Eligible pts with TN CLL/SLL aged ≥65 y were randomized 1:1 to receive 420 mg ibr daily until progression or 0.5 mg/kg clb (max 0.8 mg/kg) on days 1 and 15 of a 28-d cycle for up to 12 cycles. Pts with del17p were excluded. Pts were stratified by ECOG status and Rai stage. The primary endpoint was PFS per iwCLL 2008 criteria, with 2012 clarification for treatment related lymphocytosis. Secondary endpoints included OS, ORR, rate of hematologic improvement, and safety; longer term follow-up safety data focused on ibr. Pts in the 1115 study with progressive disease (PD) were enrolled in the PCYC-1116 extension study. At 1115 study closure, all pts in 1115 rolled over to 1116. Pts on the clb arm with PD could cross-over to ibr or investigator's choice.
Results:
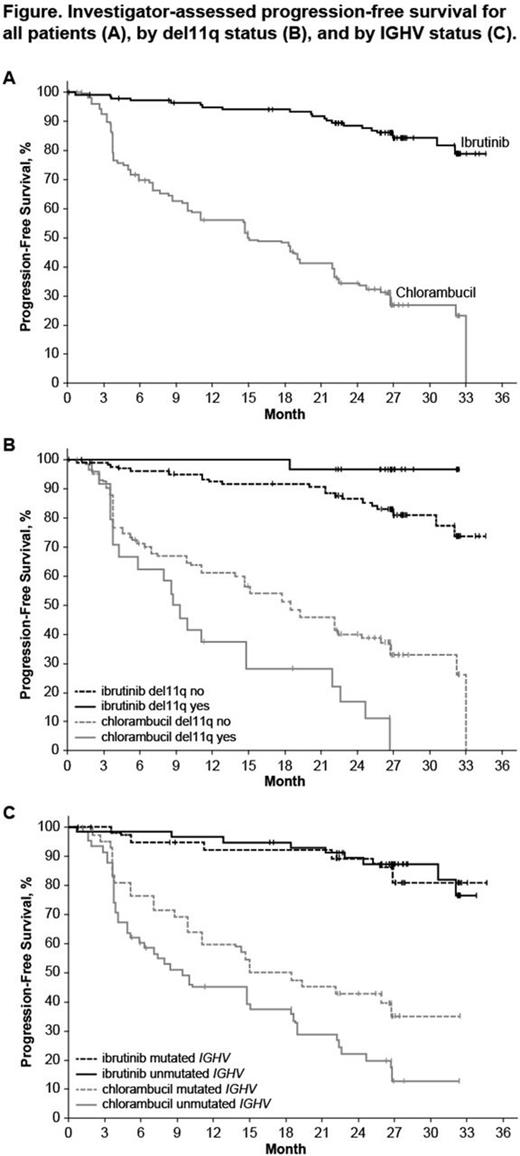
Median age of the 269 pts was 73 y (70% ≥70 y). Baseline characteristics were balanced between arms; 45% had advanced Rai stage, 20% had del11q, and 69% had comorbidities at baseline, including CIRS score >6, reduced creatinine clearance, or ECOG performance status of 2. With a median follow-up of 28.6 mo, prolongation of PFS for ibr vs clb was sustained (89% vs 34% at 24-mo; HR, 0.121; 95% CI 0.074-0.198; P<0.0001; Figure). PFS was significantly improved for ibr across high-risk subgroups, including del11q and unmutated IGHV gene (Figure). Overall, 4 of 135 pts discontinued ibr due to PD. 1 case of Richter's transformation was observed in each arm. With 41% of pts switching from clb to ibr, the OS analysis in the ITT population resulted in 2-yr survival rate estimates of 95% and 84% in the ibr and chlorambucil arms, respectively. The investigator-assessed ORR was 92% with ibr vs 36% with clb (P<0.0001). CR/CRi within the ibr arm improved from 11% at 18.4 mo to 18% with longer follow-up of 28.6-mo. Sustained hematological improvements were higher for ibr vs clb for those with anemia (90% vs 45%; P<0.0001) or thrombocytopenia (80% vs 46%; P=0.0055) at baseline. The most frequent adverse events in ibrutinib treated pts (AEs; ≥20%) are presented in the Table. Grade [Gr] ≥3 AEs in ≥5% of pts included neutropenia (12%), pneumonia (7%), anemia (7%), and hypertension (5%); AEs (any Gr) leading to dose reductions occurred in 13%. The most common reason for discontinuation was AEs (12%), with most occurring during the first yr of ibr therapy. The incidence of the common Gr ≥3 AEs in these ibr-treated pts typically decreased over time. Major hemorrhage occurred in 7% (1 Gr 2, 7 Gr 3, 1 Gr 4; 5 in first 12 mo and 4 between 1-2 y) and atrial fibrillation occurred in 10% (1 Gr 1, 7 Gr 2, 6 Gr 3) of ibr-treated pts. With a median treatment duration of 28.5 mo (range, 1-36), 79% of pts remain on first-line ibr.
Conclusions:
With a median time on study of 28.6 mo, ibr continued to have substantial efficacy, with 88% reduction in risk of progression or death. Furthermore, the quality of responses has improved over time, with 18% of CLL/SLL pts achieving a CR/CRi with single agent ibr. Treatment limiting AEs decreased in frequency with longer follow-up, with 79% of this elderly pt population continues daily ibr. Lastly, even with a high rate of cross-over in the clb arm, OS remains significantly improved for pts randomized to ibr.
Barr:AbbVie: Consultancy; Pharmacyclics, LLC, an AbbVie Company: Consultancy, Research Funding. Robak:AbbVie: Consultancy, Honoraria, Research Funding; Pharmacyclics, LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding. Owen:Gilead: Honoraria, Research Funding; Janssen: Honoraria; Lundbeck: Honoraria; Roche: Consultancy, Honoraria, Research Funding; AbbVie: Honoraria; Celgene: Honoraria; Pharmacyclics, LLC, an AbbVie Company: Research Funding. Bairey:Janssen: Consultancy. Bartlett:Gilead: Consultancy. Burger:Janssen: Consultancy, Other: Travel, Accommodations, Expenses; Portola: Consultancy; Pharmacyclics, LLC, an AbbVie Company: Research Funding; Gilead: Research Funding; Roche: Other: Travel, Accommodations, Expenses. Hillmen:Pharmacyclics: Research Funding; Janssen: Honoraria, Research Funding; Roche: Honoraria, Research Funding; Gilead: Honoraria, Research Funding; Abbvie: Research Funding. Coutre:AbbVie: Research Funding; Pharmacylics, LLC, an AbbVie Company: Consultancy, Research Funding; Janssen: Consultancy. Devereux:Roche: Consultancy, Other: Travel, Accommodations, Expenses ; Gilead: Consultancy, Other: Travel, Accommodations, Expenses, Speakers Bureau; GSK: Consultancy; Janssen: Consultancy, Other: Travel, Accommodations, Expenses, Speakers Bureau. McCarthy:Roche: Consultancy, Other: Travel, Accomodations, Expenses; Chugai: Other: Travel, Accomodations, Expenses; Celgene: Other: Travel, Accomodations, Expenses. Simpson:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees. Offner:Novartis: Consultancy; GSK: Consultancy. Zhou:AbbVie: Equity Ownership; Pharmacyclics, LLC, an AbbVie Company: Employment. Styles:AbbVie: Equity Ownership; Pharmacyclics, LLC, an AbbVie Company: Employment. James:Pharmacyclics, LLC, an AbbVie Company: Employment; AbbVie: Equity Ownership. Kipps:Roche: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Pharmacyclics, LLC, an AbbVie Company: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Speakers Bureau. Ghia:Janssen: Consultancy; Pharmacyclics, LLC, an AbbVie Company: Consultancy; Roche: Consultancy, Research Funding; GSK: Research Funding; AbbVie: Consultancy; Adaptive: Consultancy; Gilead: Consultancy, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.