Abstract
Autologous Stem Cell Transplantation (ASCT) is standard of care in relapsed diffuse large B-cell lymphoma(DLBCL) and other lymphoproliferative disorders (relapsed Hodgkin´s disease, mantle cell lymphoma (MCL) or T-cell lymphoma). BCNU, Etoposide, Ara-C, Melphalan(BEAM) is a standard conditioning regimen, but BCNU is known to be associated with interstitial pneumonia (range 2 to 20%) and an increased risk of death compared with other regimens. Therefore a less toxic conditioning protocol might improve the results in lymphoma patients. Bendamustineshowed promising results in B- and T-cell lymphoma and dose escalation is safe and feasible. Here we report promising results with bendamustinereplacing BCNU in the BEAM regimen described as Benda-EAM, previously published in a phase two dose finding study (Visani, Blood 2011).
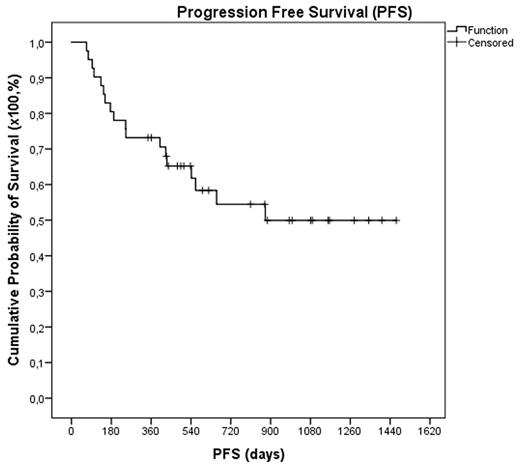
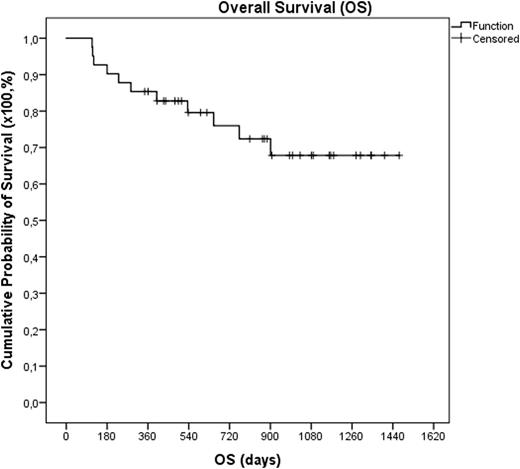
Fourty-one patients with Hodgkin´s (HL)(n=9) or Non-Hodgkin (n=32) lymphoma were consecutively treated with Benda-EAM (bendamustineon two consecutive days at a dose of 200 mg/m2 per day). Eleven patients were diagnosed with DLBCL, ten patients with MCL, six patients with follicular lymphoma (FL), three patients with T-cell lymphoma (TCL) and two patients with greyzone lymphoma (GZL). Twenty-seven patients were male and fourteen female with a median age of 52 years (range 22-71) and 25% were above the age of sixty. The median lines of previous therapies were 2 (range: 1-4). All patients had chemosensitive disease and before transplantation 34 patients (83%) were in complete (CR) and 7 (17%) in partial remission (PR). A median number of 4,20*106 CD34+ cells/kg (range: 1,60-13,30) were infused. All patients showed engraftment with a median time to achieve an absolute neutrophil count > 1*109/L of 10 days (range 8-13) and to platelets >20*109/L of 12 days (range 7-110). The median time of fever was 5 days (range: 0-15). The most common grade 3 and 4 toxicity during the whole treatment period were diarrhea (n=10), mucositis (n=7), infections (n=9) and febrile neutropenia (n=6), followedby nausea (n=4) and cardiologic toxicities (n=3). There were no pulmonary toxicities observed and no transplant related mortality occurred. After a median follow-up of 37 months 22 patients (56%) are still in CR, while 19 patients (44%) showed progression after a median time of 7 months after transplantation (range 2-29 months). Until today nine patients received an additional allogeneic transplantation. Eleven patients (27%) have died (3 DLBCL, 3 HL, 2 MCL, 1 GZL, 1 TCL and 1 FL), all due to lymphoma progression. Thus the 1- and 2-year PFS are 73.2% and 57.9% and the 1- and 2-year OVS 85.4% and 79.4%, respectively.
In conclusion Benda-EAM is feasible with a quite promising outcome. Currently an international randomized phase II trial comparing Benda-EAM with BEAM is recruiting. So far thirty-five of 110 planned patients are randomized and first results are expected for 2018.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.