Abstract
Backgound: Sinusoidal obstruction syndrome [SOS, also known as hepatic veno-occlusive disease (VOD)] is frequent after HSCT and may have a mortality rate of up to 85%. Defibrotide has shown efficacy not only in the treatment of established SOS (Richardson et al. Blood 1998:92;737 and 2016:127;1656) but also in SOS prevention in children (prospective study: Corbacioglu et al. Lancet 2012:379;1301) as well as in adults (several retrospective studies).
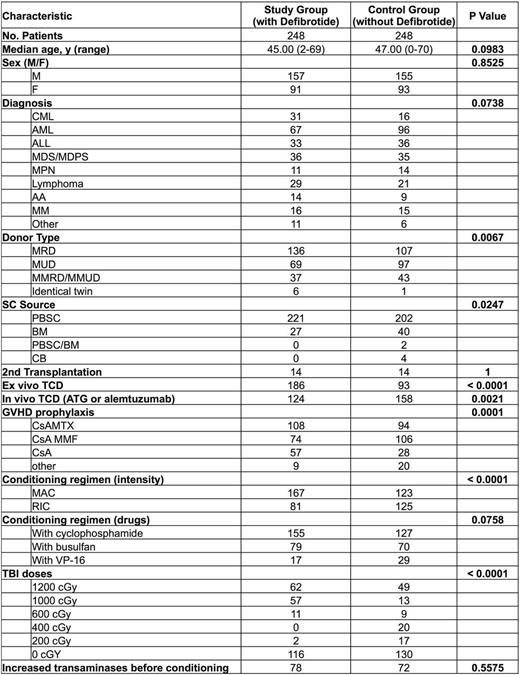
Patients and methods: Between 1999 and 2009, we gave defibrotide intravenously to 248 successive patients transplanted for hematological diseases starting at day -7 up to day +20 post-transplantation (dose range 800-2400 mg/d) in combination with heparin. We previously published the data of the 52 first patients compared to 52 historical controls who were transplanted just prior to the study period. We now expand the study with 196 additional patients treated successively with defibrotide prophylaxis while adding patients transplanted after 2010 when patients did not receive defibrotide as prophylaxis anymore (2011-2015) to the control group. The characteristics of patients are shown in Table 1.
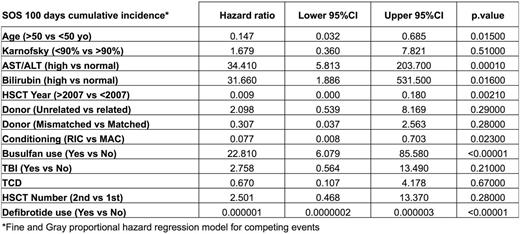
Results: Median follow-up for the study group was 10 (range 2-16) years and for the control group 2.7 (range 1-18) years. None of the 248 patients in the defibrotide group developed SOS (Baltimore criteria). The 100 day cumulative incidence (CI) of SOS was 0% in the defibrotide group as compared to 4.8% (95%CI 2.6-8%) in the control group, p=0.00046. The day 100 event free survival (EFS) where an event was defined as the 1st occurrence of one of the following: SOS, acute graft versus host disease (GvHD) ≥2, relapse or death, was not significantly different with 60% (95%CI 54%-66%) in the defibrotide group vs 53% (95%CI 47%-59%) in the controls, p=0.165, but the one year EFS was statistically different with 38% (95%CI 32%-44%) vs 28% (95%CI 22%-34%), p=0.00969. The 100 day CI of acute GVHD was not significantly different between the two groups [27% (95%CI 22%-33%) in the defibrotide group vs 29% (95%CI 24%-35%) in the control group, p= 0.707] while the 1 year acute GvHD CI was significantly reduced in the defibrotide group [31% (95%CI 25%-37%)] compared with the control group [42% (95%CI 36%-48%), p=0.026]. The one year overall survival (OS), relapse incidence (RI) and non-relapse mortality (NRM) were not statistically different being respectively 73% (95%CI 67%-78%) vs 65% (95%CI 59%-71%), p=0.0704, 32% (95%CI 27%-38%) vs 28% (95%CI 22%-34%), p=0.331 and 14% (95%CI 10%-18%) vs 19% (95%CI 14%-24%), p=0.148. Multivariate analysis, performed taking into account clinical factors known to influence the risk of SOS, confirmed the favorable impact of defibrotide on 100 day SOS CI [HR 7.5x10-7 (95%CI 1.8x10-7-3.2x10-6), p<0.00001] (Table 2). Conversely, multivariate analysis failed to confirm the impact of defibrotide on 1 year EFS or acute GvHD CI.
Conclusion: To the best of our knowledge, this is the largest study on prophylaxis of SOS with defibrotide and it suggests that this drug has a potential benefit in the prevention of this liver complication. Our retrospective study needs to be confirmed in a prospective randomized trial.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.