Abstract
Introduction:
Multiple studies have shown that tyrosine kinase inhibitor (TKI), usually imatinib (IM), can be successfully discontinued in chronic myeloid leukemia (CML) patients, achieving a 40-50% treatment free remission (TFR) rate. However, the remaining 60% of patients require reintroduction of TKI therapy. We have designed this study to answer the question if a second generation TKI, i.e. dasatinib, should be used after failing the first attempt of TKI discontinuation with IM. This prospective study attempts to evaluate whether the use of dasatinib after failure of a 1st attempt of TKI discontinuation with IM could improve TFR rate after a 2nd attempt of TKI discontinuation.
Methods and materials:
This is a prospective clinical trial (BMS CA180-543, Clinicaltrial.gov NCT#02268370) including all major CML centers across Canada (n=12). The primary objective is to determine the proportion of patients who remain in molecular remission (defined as maintaining ≥MR4.0) after dasatinib discontinuation following achieving ≥MR4.5. The present study has 3 phases: 1) IM discontinuation phase, 2) dasatinib rechallenge phase, 3) dasatinib discontinuation phase. A total of 135 patients is planned to be enrolled over 18-24 months.
Key inclusion criteria include: 1) CML in chronic phase (CP), 2) total duration of IM therapy of minimum of 3 years, 3) total duration of MR4.5 or deeper response over 2 years with 2 consecutive confirmed MR4.5 or deeper response at the central lab with 3 months interval. Key exclusion criteria include prior allogeneic stem cell transplant or prior accelerated or blastic phase CML. Molecular recurrence was defined as an increase in BCR-ABL transcript level above MR4.0, on at least two consecutive occasions, or a single increase in BCR-ABL transcript level above MR3.0. Dasatinib is started at a dose of 100mg daily once molecular recurrence is confirmed.
Results:
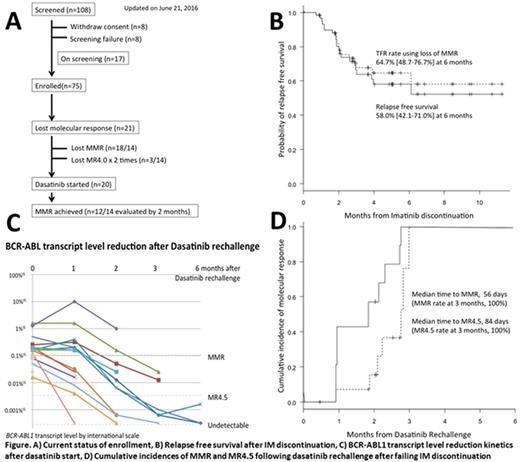
The study was launched on March 2015. As of June 22 2016, 110 patients were entered into screening phase of whom 16 patients were not qualified due to 1) fluctuating transcripts (n=8) or 2) consent withdrawal (n=8). Two patients withdrew consent after losing their molecular response following IM discontinuation before starting dasatinib. Finally 75 patients were enrolled into IM discontinuation phase with 17 additional patients on screening in awaiting enrollment (Figure A).
Of 67 patients evaluable for molecular relapse, 21 patients (31.3%) lost molecular response defined as loss of major molecular response (MMR; n=18) and loss of MR4 on 2 consecutive tests (n=3). The 6-month relapse free survival (RFS) rate was estimated as 58.0% (42.1-71.0%), while TFR rate using loss of MMR as an event was 64.7% (48.7-76.7%) at 6 months (Figure B)
Of 21 patients who lost molecular response, 20 patients underwent dasatinib rechallenge phase, 14 of which were treated with dasatinib for at least 1 month and evaluated at least once with monthly BCR-ABL transcript monitoring (Figure C). Twelve patients achieved MMR at a median time of 56 days. The median time to achieve MR4.5 was 84 days. The incidence of MMR and MR4.5 at 3 months was 100% each (Figure D).
Cox's proportional hazard regression model suggested strong correlation of RFS with total duration of IM therapy prior to IM discontinuation (p=0.001), duration of MR4 maintenance (p=0.004) or MR4.5 maintenance (p=0.006) prior to IM discontinuation, but not with time to achieve MR4 (p=0.289) or MR4.5 (p=0.330). Recursive partitioning method also defined the best cutoff for those variables: The group treated with IM for over 8.9 years (n=35) had RFS rate 81.1% vs 21.0% in those treated for less than 8.9 years (n=32; p<0.001, HR 0.125). The group who maintained MR4 for more than 7.7 years (n=29) had RFS rate 82.3% vs 36.3% in others (n=37; p=0.001, HR 0.192), while those who maintained MR4.5 for more than 7.9 years (n=21) had RFS rate 89.5% vs 39.2% in others (n=44; p=0.003, HR 0.141).
Conclusion:
Preliminary results of this Canadian TKI discontinuation trial show a RFS rate of 58-65% after IM discontinuation in CP-CML patients who attained MR4.5 for over 2 years, similar to other TKI discontinuation studies. Dasatinib can be safely administered in CML patients who lost molecular response after IM discontinuation with 100% of MMR rate at 3 months. Prolonged duration of IM treatment, or duration of MR4/MR4.5 maintenance with IM therapy could predict the chance of TFR success, but not time to achievement of MR4/MR4.5.
Bence-Bruckler:Lundbeck: Membership on an entity's Board of Directors or advisory committees. Savoie:Amgen: Consultancy; Celgene: Consultancy; Pfizer: Consultancy; BMS: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Speakers Bureau; Jazz: Consultancy; Lundbeck: Consultancy. Busque:Pfizer: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau. Delage:BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding. Laneuville:BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Paladin: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Liew:BMS: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Xenocostas:Janssen Inc.: Research Funding. Kamel-Reid:BMS: Research Funding. Leber:BMS Canada: Honoraria, Research Funding, Speakers Bureau; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract