Abstract

Introduction: Venous thromboembolism (VTE) is a significant cause of morbidity in patients with hematologic malignancy who undergo hematopoietic cell transplantation (HCT); however, clinically significant bleeding is not uncommon in this setting and anticoagulation is often contraindicated during prolonged periods of severe thrombocytopenia. This study aims to determine the relative risks of continuing versus temporarily withholding therapeutic anticoagulation during periods of chemotherapy-induced thrombocytopenia in patients who undergo autologous HCT.
Methods: Adult patients with hematologic malignancies who underwent first autologous HCT at our institution between 2006 and 2015 were selected. Among those, patients with VTE (as identified by ICD9 code and confirmed by chart review) that occurred in the year preceding transplant were selected as the study population. Patients were allocated into two cohorts at the onset of thrombocytopenia based on whether they continued anticoagulation using a high platelet transfusion threshold (50k), or whether they temporarily withheld anticoagulation using a standard platelet transfusion threshold (10k) and restarted treatment upon platelet engraftment. Patient characteristics and VTE risk factors were captured, depicted as number (percentage) and median (interquartile range), and compared using Fisher's exact and rank sum tests.
Primary outcomes included rate of VTE extension/recurrence (assessed by appropriate imaging modality) and major bleeding (defined as grade 3 or 4 bleeding by WHO criteria) by 30 days. Secondary outcomes included clinical bleeding (grade 2-4), overall mortality, median number of platelet and red blood cell (RBC) transfusion, median time to neutrophil and platelet engraftment. Pre-defined exploratory subgroup analysis was performed. Logistic regression, Cox regression, and linear regression models without adjustment were generated for outcomes where a P value of < 0.05 was considered significant (Stata 14.1).
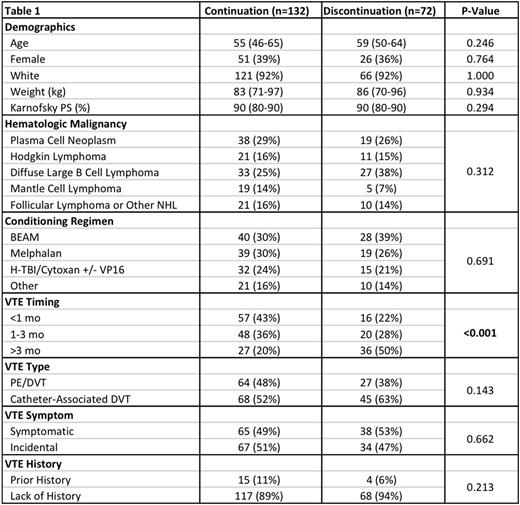
Results: Among 1,631 patients who underwent first autologous HCT between 2006 and 2015, 204 patients developed VTE (12.5%) in the year preceding transplant. The median duration of thrombocytopenia, defined as the period during which the platelet < 50k, was 12 days. The median follow-up period was 359 days and only 4% of patients were lost to follow up prior to 30 days. In addition to routine clinical assessment for VTE and bleeding, repeat imaging surveillance was done in 55% of patients prior to discharge from the transplant service. Except for the timing of prior VTE occurrence, there were no significant differences in baseline characteristics between the cohort that continued anticoagulation (N=132) and the cohort that discontinued anticoagulation (N=72) (Table 1).
There were no significant differences in the rate of VTE extension/recurrence or major bleeding between the treatment groups by 30 days (Table 2). The rates of VTE in both groups were low (1 new pulmonary embolism and 2 new catheter-associated thromboses). There was 1 fatal spontaneous retroperitoneal bleeding (grade 4) in the cohort that continued anticoagulation. The number of platelet transfusions was significantly higher in the patients who continued anticoagulation. Further comparison of the 2 cohorts by different subgroups did not reveal significant interactions (Figure 1, all interaction P values > 0.300).
Conclusions: In patients undergoing HCT for hematologic malignancy who also have VTE, continuation of anticoagulation (compared to temporary cessation) during chemotherapy-induced thrombocytopenia is associated with increased platelet utilization but no significant difference in the rate of VTE extension/recurrence. The low rate of recurrent thrombosis among patients who discontinued anticoagulation during pre-engraftment thrombocytopenia suggests that temporary interruption of anticoagulation may be the better option for selected patients who face this difficult clinical situation.
Gopal:Seattle Genetics: Research Funding. Garcia:Daiichi Sankyo: Consultancy, Research Funding; Pfizer: Consultancy; Boehringer Ingelheim: Consultancy; BMS: Consultancy; Janssen: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract