Abstract
The mechanisms underlying the PF4/heparin immune response are poorly understood. In recent studies, we showed that PF4/heparin complexes, but not PF4 alone or heparin alone, activate complement (C') in a heparin-dependent manner and lead to selective binding of C'-coated antigen (PF4/heparin complexes) to B cells via CD21. In additional studies, we showed that heparinized patients have circulating B cells with C'-coated PF4/heparin complexes (Khandelwal, Blood 2016). To investigate the mechanism by which PF4/heparin complexes activate complement, we performed studies in whole blood using previously described methods assessing binding of C'-fixed PF4/heparin complexes to B cells in whole blood. We incubated blood with inhibitors or conditions known to selectively block specific complement activation pathways, followed by incubation with PF4 and heparin for 1 hour (hr) at 370 C. Binding of PF4/heparin complexes and C' fragments to B-cells was determined by flow cytometry using the murine monoclonal antibody to PF4/heparin complexes (KKO) and antibodies to specific C' activation products (Khandelwal, Blood 2016).
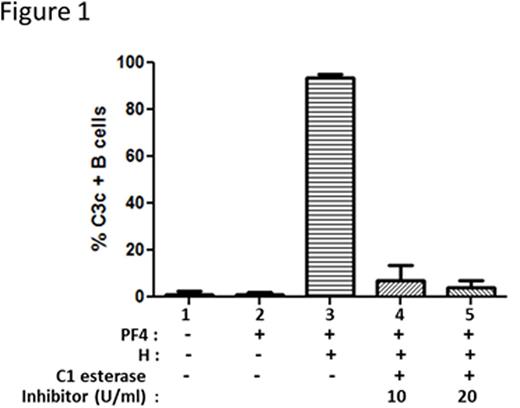
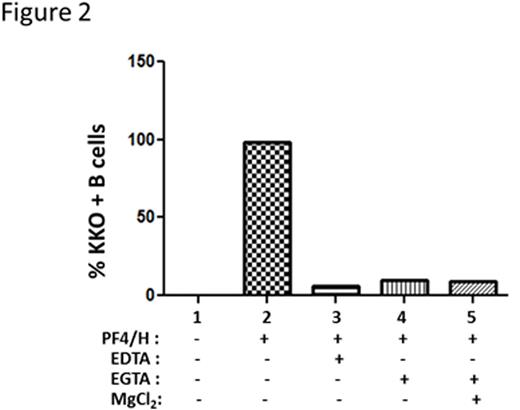
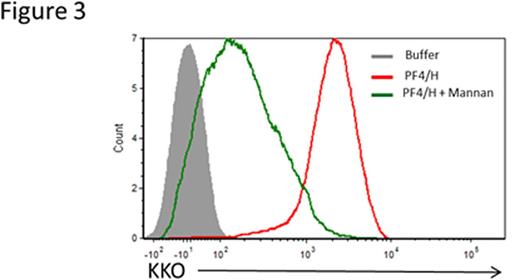
To distinguish activation by classical and lectin pathways from alternative pathway of activation, we incubated blood with C1 esterase inhibitor, a protein that inhibits C' activation by the classical and lectin pathways. As shown in Figure 1, whole blood incubated with PF4/heparin is associated with C' activation as measured by C3c binding to B cells (Figure 1, column 3), but not if blood is incubated with buffer or PF4 alone (Figure 1, columns 1 & 2). With the addition of C1 esterase inhibitor (10 or 20 U/ml) prior to incubation with PF4/heparin complexes, we noted a dose-dependent reduction in C' activation (>85% reduction with 10 and 20 U/ml) suggesting that C' activation by PF4/heparin complexes occurs via the classical or lectin pathways. To further confirm that the alternate pathway is not involved in PF4/heparin mediated C' activation, we used sensitivity of the alternative pathway to Mg2+ using differential chelation with EDTA and EGTA. EDTA, a chelator of both Ca2+ and Mg2+, inhibits complement activation by all three pathways, whereas EGTA, which preferentially sequesters Ca2+ over Mg2+, permits alternative pathway activation. As shown in Figure 2, incubation of PF4/heparin complexes without chelators allowed for maximal antigen binding to B cells (~100%), while incubation with EDTA (Figure 2, column 3) abolished antigen binding, as did EGTA with and without additional Mg2+ supplementation. We next investigated the role of the classical pathway, a pathway triggered by immune complex mediated-binding and activation of C1. By flow cytometry, we were unable to show C1q deposition although we were able to demonstrate C3c/C4c deposition on antigen-coated B cells from healthy donors (data not shown). We also showed that C' activation by PF4/heparin complexes was preserved in human serum low in immunoglobulins (IgG and IgM) as well as plasma from mMT mice lacking circulating immunoglobulins (data not shown). To investigate the role of the lectin pathway, we performed competitive inhibition assays by incubating whole blood with PF4/heparin complexes in the presence of mannan. As shown in Figure 3, we show that mannan (500 mg/mL) inhibited binding of antigen to B cells. In other studies, we show that an anti-MBL antibody partially reduced binding of KKO/C' to B cells. Together, these preliminary studies demonstrate a lack of involvement of the classical and alternative pathways in C' activation and indicate a contribution by the lectin pathway in C' activation by PF4/heparin complexes. Further studies are underway to elucidate the role of the lectin pathway in complement activation by PF4/heparin complexes. Insights from these studies will lead to novel interventions that can block the initial steps of immune activation by heparin.
Arepally:Biokit: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.