Abstract
Background: Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous disease with distinct survival differences according to molecular subtype with superior outcomes in patients with the germinal center B cell-like (GC) subtype as compared to those with the activated B cell-like (ABC) subtype. Efficacy data for single-agent ibrutinib in patients with relapsed/refractory (r/r) DLBCL are limited to a single clinical trial of 80 patients. In that study, higher response rates were observed for r/r ABC-DLBCL compared to GC-DLBCL (37% vs. 5%), when assigned by gene expression profiling (GEP). The response rate of those with unknown/unclassifiable DLBCL was 22%. Despite biologic rationale for selective cytotoxicity of ibrutinib for ABC-DLBCL, it is not clear that such preferential activity will be observed when subtyping based on immunohistochemical (IHC) staining is used, as the correlation with subtype determined by GEP and IHC is imperfect. Furthermore, GEP is time consuming and expensive so IHC is used in clinical practice to differentiate GC from non-GC, the latter of which includes both ABC and unclassifiable DLBCL. We retrospectively analyzed outcomes of patients with r/r DLBCL treated with ibrutinib at a number of large academic medical centers.
Methods: We reviewed medical records of all patients with DLBCL treated with ibrutinib at five U.S. tertiary-care cancer centers from 2013 to 2016. We included patients with de novo DLBCL as well as those transformed from indolent lymphoma if the ibrutinib was given for the DLBCL histology. Patients were excluded if they received ibrutinib for ≤ 14 days. Molecular subtype (GC vs non-GC) was determined by local pathology findings and/or the investigator's application of the Hans algorithm. Categorical variables were compared between groups using the Chi-square test. Outcomes were calculated from the date of initiation of ibrutinib. Overall survival (OS) and progression-free survival (PFS) were estimated using the Kaplan-Meier method and compared using the log-rank test.
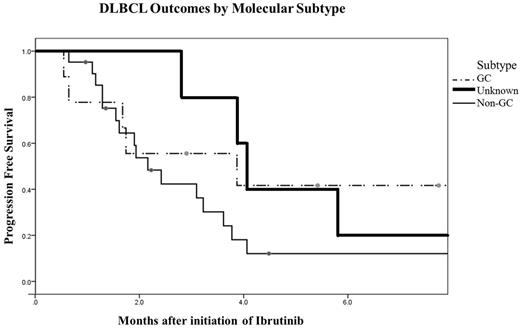
Results: Thirty five patients met inclusion criteria (27 de novo and 8 transformed DLBCL). The median age at diagnosis was 61 years (range 38-88) with 66% men.By Hans IHC criteria, there were 21 cases of non-GC, 9 GC and 5 were unknown.The median number of treatments prior to ibrutinib was 3 (range 1-8). 30% of patients had undergone prior autologous stem cell transplant. Characteristics including age, gender, transformed versus de novo, relapsed versus refractory, number of prior therapies, and prior use of transplant did not significantly differ between subgroups. The overall response rate (ORR) to ibrutinib was 29% with 4 patients achieving a complete response (CR) and 6 achieving a partial response (PR). When evaluated by subtype assigned by IHC, GC-DLBCL patients had an ORR of 44% and non-GC-DLBCL patients had an ORR of 19%. There was no significant difference in the rates of CR, PR, stable disease, or progressive disease between the subtypes (p=0.185) or between de novo ortransformed disease (P = 0.114). The median progression-free survival (PFS) was comparable for patients with the GC, non-GC, and unknown subtype (3.9, 2.2, and 4.1 months, respectively, P = 0.382, Figure). The median overall survival (OS) was longer for patients with the GC subtype (10.5 months) compared to 5.5 months for patients with the non-GC subtype, and 9.7 months for those with unknown subtype but this difference was not statistically significant (P=0.564).
Figure: Progression Free Survival of r/r DLBCL Patients Treated with Ibrutinib, based on Hans Algorithm Subtype
Conclusions: Responserates to single agent ibrutinib in the GC and non-GC subtypes of r/r DLBCL do not appear different when using Hans algorithm to assign subtypes. PFS and OS were modest in both groups and not statistically different. In conclusion, until GEP or other molecular technologies such as Nanostring are in more widespread use for routine subtyping of DLBCL, caution is advised when selecting patients for subtype-specific therapy, as clinical outcomes for patients receiving ibrutinib may not differ by cell of origin as determined by IHC.
Reddy:celgene: Membership on an entity's Board of Directors or advisory committees; KITE: Membership on an entity's Board of Directors or advisory committees; GILEAD: Membership on an entity's Board of Directors or advisory committees; INFINITY: Membership on an entity's Board of Directors or advisory committees. Shadman:Pharmacyclics: Honoraria, Research Funding. Smith:Spectrum: Honoraria; Celgene: Honoraria; Abbvie: Research Funding; Genentech: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.