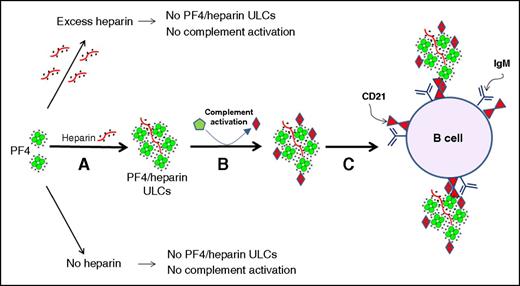
In this issue of Blood, Khandelwal et al show that B cells bind platelet factor 4 (PF4)/heparin complexes in a non–antigen-specific fashion, mediated by complement activation and resulting complex binding via B-cell complement receptors (CD21).1
Activation of complement by PF4/heparin complexes and binding to B cell CD21. (A) PF4 and heparin interact over a narrow range of molar ratios to generate ULCs. PF4 alone or PF4 with excess of heparin do not make ULCs. (B) ULCs activate complement and bind complement activation products (C3/C4). (C) Complement-coated ULCs bind to a B cell via CR2/CD21. See Figure 7 in the article by Khandelwal et al that begins on page 1789.
Activation of complement by PF4/heparin complexes and binding to B cell CD21. (A) PF4 and heparin interact over a narrow range of molar ratios to generate ULCs. PF4 alone or PF4 with excess of heparin do not make ULCs. (B) ULCs activate complement and bind complement activation products (C3/C4). (C) Complement-coated ULCs bind to a B cell via CR2/CD21. See Figure 7 in the article by Khandelwal et al that begins on page 1789.
Immunization triggered by heparin is a common event. In some clinical settings, such as cardiac surgery performed using heparin anticoagulation, 50% to 80% of patients develop an immune response detectable ∼1 to 2 weeks post-exposure.2,3 The antibodies recognize multimolecular complexes of (polyanionic) heparin bound to (cationic) PF4.4 In most patients, the immune event is inconsequential; however, for some, highly pathogenic antibodies activate platelets and monocytes, resulting in an intensely prothrombotic reaction characterized by falling platelet counts and a high risk of thrombosis, a disorder known as heparin-induced thrombocytopenia (HIT).5 The high frequency of heparin-induced immunization remains one of the most perplexing aspects of this important clinical problem.
Khandelwal et al now report that >90% of B cells obtained from normal individuals bind PF4/heparin complexes. This effect occurred rapidly (saturation within 30 minutes), was seen with B cells rather than other circulating leukocytes (2% or lower binding for T cells, monocytes, and neutrophils), and was not seen with PF4 alone but only occurred when PF4 was bound to heparin, and particularly at concentrations of PF4 and heparin that optimized the formation of ultra-large complexes (ULCs) comprising these 2 constituents. Such a high frequency of binding of PF4/heparin complexes to B cells cannot represent a specific effect of surface antibodies, and indeed these workers further showed that the mechanism of binding involves a noncognate antigen type of mechanism, namely the activation of complement by formation of PF4/heparin complexes, followed by the binding of PF4/heparin/complement complexes to B cells via CD21 (ie, the B-cell complement receptor 2 [CR2]).
These observations could help explain the unusually high frequency of the heparin-induced immune response. After all, if most B cells bind PF4/heparin/complement complexes in a noncognate fashion, it would seem plausible that this would stimulate a subset of B cells that bear surface immunoglobulins (Igs) that have any degree of affinity to PF4/heparin complexes, including low or moderate affinity interactions characteristic of HIT antibodies.6 Indeed, the binding of complement-coated antigen to CR2/CD21 enhances immunogenicity of some antigens by several orders of magnitude.7 Although further progression of the anti-PF4/heparin immune response likely requires other contributors such as T-cell involvement,8 the initial interaction of PF4/heparin complexes formed in vivo with most circulating B cells could explain the high frequency of this immune response.
Of course, not all heparin-exposed patients form anti-PF4/heparin antibodies, and not all antibody-positive patients develop clinical HIT.9 Could patient-specific differences in B-cell binding of PF4/heparin/complement complexes be important in explaining aspects of this clinical heterogeneity? Here, Khandelwal et al showed that among 16 patients receiving heparin at usual therapeutic doses, approximately one-third (6/16, or 37%) exhibited binding of PF4/heparin complexes to their B cells. This phenomenon was heparin-dependent, because it was only demonstrable after commencing the heparin infusion; moreover, at very high heparin concentrations (sufficient to raise the activated partial thromboplastin time to >300 seconds), the antigen complexes no longer bound to B cells. These fascinating observations point to future investigations: is the subset of patients whose B cells bind PF4/heparin complexes the same patient population that subsequently form high levels of anti-PF4/heparin antibodies? Unlike the majority of immune-mediated adverse drug reactions affecting blood cells, which are rare, the anti-PF4/heparin immune response is common, and even clinical HIT occurs often enough that a future study that systematically evaluates the predictivity of PF4/heparin binding to B cells among individuals patients in determining their subsequent immune response, would likely be feasible.
As an aside, the authors also noted that protamine (PRT)/heparin complexes exhibited similar features of preferential B-cell binding; although not the focus of their current paper, these observations could provide insights into the high frequency of formation of anti-PRT/heparin complex antibodies, a recently recognized immune response that, like the anti-PF4/heparin immune response, is common, rapid, and relatively transient.10
The high degree of binding of PF4/heparin complexes to B cells from many normal individuals suggested to Khandelwal et al that a non–antigen-specific mechanism must be operative, and indeed the authors showed that PF4/heparin binding to B cells in vitro required the presence of complement. Further, B cells isolated from heparin-treated patients showed that for those whose B cells bound PF4/heparin complexes, C3 and C4 also could be detected on their B cells, whereas those patients whose B cells did not bind PF4/heparin complexes, these complement system components could not be detected either. Using a variety of techniques, the authors convincingly showed that binding of PF4/heparin/complement complexes occurred via CD21 on B cells, otherwise known as CR2.
The authors propose the following early steps during the pathogenesis of the anti-PF4/heparin immune response. Administration of pharmacologic heparin displaces PF4 from platelets and endothelium, raising its concentration ∼10-fold, and leading to the formation of PF4/heparin ULCs that bind complement and thereby bind preferentially to B cells via CR2 (see figure). This general mechanism could explain several of the known features of the anti-PF4/heparin immune response, including its high frequency (because near ubiquitous binding to B cells in many individuals seems likely to trigger B-cell response against the PF4/heparin complexes) and greater frequency of immunization triggered by unfractionated heparin (UFH) vs low molecular weight heparin (LMWH) (because the authors found lower dose requirements for UFH vs LMWH for producing ULCs capable of activating complement). These exciting studies represent a real breakthrough in understanding how it is that heparin can trigger so often an immune response, and provides further insights into how the innate and adaptive immune systems interact in explaining certain adverse drug effects.
Conflict-of-interest disclosure: T.E.W. has received lecture honoraria from Pfizer Canada and Instrumentation Laboratory, royalties from Taylor & Francis (Informa), consulting fees and research funding from W.L. Gore and Instrumentation Laboratory, and has provided expert witness testimony relating to HIT.