In this issue of Blood, Cicalese and colleagues report excellent medium-term survival and immune reconstitution outcomes for patients with adenosine deaminase (ADA)–deficient severe combined immunodeficiency (SCID) treated with a retroviral gene vector.1
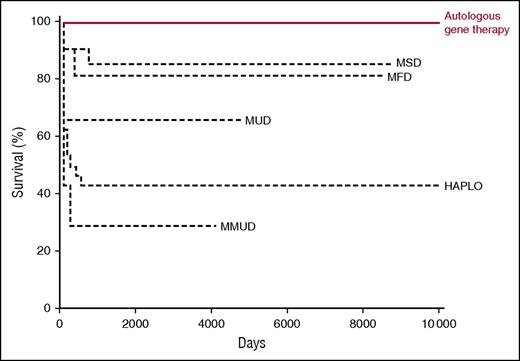
Kaplan-Meier curve showing overall survival in relation to donor source after hematopoietic stem cell transplantation and gene therapy. HAPLO, haploid-identical T-cell–depleted donor; MFD, matched family donor; MMUD, mismatched unrelated donor; MSD, matched sibling donor; MUD, matched unrelated donor. Professional illustration by Somersault 18:24, adapted from Cicalese et al1 and Hassan et al.9
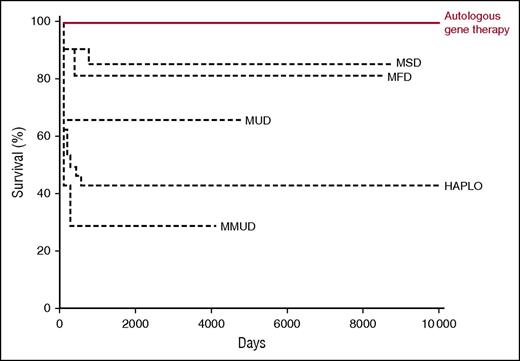
Kaplan-Meier curve showing overall survival in relation to donor source after hematopoietic stem cell transplantation and gene therapy. HAPLO, haploid-identical T-cell–depleted donor; MFD, matched family donor; MMUD, mismatched unrelated donor; MSD, matched sibling donor; MUD, matched unrelated donor. Professional illustration by Somersault 18:24, adapted from Cicalese et al1 and Hassan et al.9
Almost 60 years ago, the syndrome of Swiss-type agammaglobulinemia was described. Patients were agammaglobulinemic with absolute T lymphocytopenia and developed persistent viral gastrointestinal and respiratory tract infection; the disease was uniformly fatal by the age of 12 to 18 months. Following unsuccessful thymic transplant, one of the first patients to undergo therapeutic allogeneic hematopoietic stem cell transplantation was a patient with severe combined immunodeficiency, as “agammaglobulinemia and alymphocytosis” was renamed. Subsequently, almost 20 genetic forms of SCID have been described; they lead to failure of T-lymphocyte development, with variable effects on B-lymphocyte and natural killer cell development.2 Since those early days, hematopoietic stem cell transplantation has become the treatment of choice, with improving success.3 Many developments have occurred since then, including the development and refinement of T-lymphocyte–depleted hematopoietic stem cell transplantation and the introduction of newborn screening,4 as well as a greater understanding of T- and B-lymphocyte developmental biology. Outcome has changed dramatically, from universally fatal to over 90% survival in well, noninfected newborns. In the late 1990s, the concept of replacing the faulty gene in recipient hematopoietic stem cells, rather than replacing recipient with donor stem cells, was born from careful observation of rare wild-type revertants.5 Initial trials using retroviral vectors showed efficacy in curing X-linked SCID due to mutations in IL2RG but were complicated by random insertion of the vector next to proto-oncogenes in some cells, leading to oncogenesis and lymphoproliferative disease.6 New trials using lentiviral vectors are ongoing and to date have not developed lymphoproliferative complications.
Adenosine deaminase deficiency is an unusual form of SCID in that it is a metabolic defect of purine metabolism that causes an accumulation of deoxyadenosine and deoxyadenosine triphosphate, metabolites that are toxic to developing lymphocytes.7 Patients born with profoundly reduced or absent enzyme activity present with classical SCID, as well as associated cognitive and auditory impairment. Unlike most other forms of SCID, enzyme replacement with polyethylene glycol–modified bovine adenosine deaminase (PEG-ADA) is available, but lifelong treatment is required, is expensive, and only partially restores immune function, and long-term immune responses to PEG-ADA can impair efficacy.8 A previous study, which documented outcome of allogeneic hematopoietic stem cell transplantation, described significantly worse outcomes when haploidentical donors were used compared with genoidentical donors.9 Given that most patients do not have an identical sibling donor available and that enzyme replacement is suboptimal, ADA-deficient SCID was also an ideal model for gene therapy. At the same time that the X-linked SCID trials were being developed, retroviral vector gene therapy was introduced for ADA deficiency. Initial reports were encouraging, but in this issue of Blood, Cicalese and colleagues report on the medium-term survival, immune reconstitution, adverse effects, growth, and development in a cohort of 18 patients who received autologous CD34+-enriched cell fractions that contained CD34+ cells transduced with a retroviral vector encoding the human ADA complementary DNA sequence, following low-dose busulfan conditioning. With a median follow-up period of 6.9 years (range, 2.3-13.4 years), survival is 100%, results that compare favorably with PEG-ADA enzyme replacement and allogeneic hematopoietic stem cell transplantation (see figure). Most patients demonstrated restoration of T lymphocytes to normal or just below normal ranges, with evidence of thymopoiesis, sustained gene-marked circulating T lymphocytes, normal mitogen-induced T-lymphocyte proliferation, B-lymphocytopoiesis (albeit with lower-than-normal circulating B-lymphocyte numbers), and, in some, antibody production to killed and live vaccine antigen and to natural varicella-zoster virus infection. Importantly, although lymphocyte numbers were not completely normalized in all patients, there was evidence of purine metabolite detoxification comparable to that seen in allogeneic hematopoietic stem cell transplantation. The rate of severe infections significantly decreased after gene therapy, and although there was no clear improvement in growth or neurological events, this would not be expected for a hematopoietic stem cell procedure. In 3 patients, the procedure was not considered successful, and although gene transduction was documented, PEG-ADA was recommenced due to poor immune reconstitution; 2 patients have subsequently undergone successful matched sibling donor hematopoietic stem cell transplantation. Critically, although there was evidence of random vector insertion at or near oncogene sites, persisting in the medium-term, no adverse events have been demonstrated at the laboratory or clinical level to date.
This study is important for several reasons. Firstly, the participants in the trial included patients who had previously failed haploidentical hematopoietic stem cell transplantation or who had been on PEG-ADA for several months to years and were successfully treated, unlike early gene therapy trials for ADA deficiency. Secondly, the overall results compare extremely favorably to hematopoietic stem cell transplantation, including when using genoidentical sibling donors. Thirdly, despite using similar retroviral vectors to other primary immunodeficiency (PID) gene therapy trials in which mutagenesis was observed, no oncogenic effects have been observed in over 131 follow-up patient-years.
The concept of curing inborn errors by use of genetically modified autologous cells is attractive, although longer-term follow-up studies will be required to determine durability of immune reconstitution and immune competence, ongoing safety of gene-transduced cells, and long-term effects of even low-dose alkylating agents such as busulfan. With the issuing of a positive opinion recommending marketing authorization from the Committee for Medicinal Products for Human Use of the European Medicines Agency for an ADA gene therapy vector,10 ADA deficiency may be the first primary immunodeficiency in which gene therapy becomes the “standard of care.” From a disease that, 50 years ago, was universally fatal, and for which there was no available treatment, we are moving to an era where almost 100% survival is expected using gene-corrected autologous cells to provide a lifelong cure. For gene therapy in the field of primary immunodeficiency, this is not the beginning of the end but, truly, the end of the beginning.
Conflict-of-interest disclosure: The author declares no competing financial interests.