Abstract
The impact of transfusing fresher vs older red blood cells (RBCs) on patient-important outcomes remains controversial. Two recently published large trials have provided new evidence. We summarized results of randomized trials evaluating the impact of the age of transfused RBCs. We searched MEDLINE, EMBASE, CINAHL, the Cochrane Database for Systematic Reviews, and Cochrane CENTRAL for randomized controlled trials enrolling patients who were transfused fresher vs older RBCs and reported outcomes of death, adverse events, and infection. Independently and in duplicate, reviewers determined eligibility, risk of bias, and abstracted data. We conducted random effects meta-analyses and rated certainty (quality or confidence) of evidence using the GRADE approach. Of 12 trials that enrolled 5229 participants, 6 compared fresher RBCs with older RBCs and 6 compared fresher RBCs with current standard practice. There was little or no impact of fresher vs older RBCs on mortality (relative risk [RR], 1.04; 95% confidence interval [CI], 0.94-1.14; P = .45; I2 = 0%, moderate certainty evidence) or on adverse events (RR, 1.02; 95% CI, 0.91-1.14; P = .74; I2 = 0%, low certainty evidence). Fresher RBCs appeared to increase the risk of nosocomial infection (RR, 1.09; 95% CI, 1.00-1.18; P = .04; I2 = 0%, risk difference 4.3%, low certainty evidence). Current evidence provides moderate certainty that use of fresher RBCs does not influence mortality, and low certainty that it does not influence adverse events but could possibly increase infection rates. The existing evidence provides no support for changing practices toward fresher RBC transfusion.
Background
Red blood cell (RBC) transfusion for surgical and critically ill patients remains a vital, and for some life-threatening situations, the only medical strategy for treating clinical symptoms related to bleeding and anemia. Nevertheless, recent randomized trials have suggested that restrictive transfusion thresholds (eg, transfusion at a hemoglobin concentration 7.0-9.0 g/dL [or 70-90 g/L] vs 10 g/dL) results in better outcomes than do higher thresholds.1-4 These counterintuitive results have fueled speculation regarding possible explanations. One explanation is the possible deleterious effects of transfusion of RBCs that have undergone relatively longer storage times.
Studies in controlled animal models have demonstrated that longer storage of RBCs leads to morphologic changes that could have a deleterious impact on microvascular perfusion and thus oxygen delivery. These changes include changes to the cell shape and membrane, an increase in adhesiveness, a decline in flexibility (rigidity that can hamper blood flow hemodynamics), and reductions in capillary flow.5-9 Further, older blood is associated with release of free iron that may predispose to vascular dysfunction, thrombosis, and nosocomial infections.5-9
Finally, the storage medium could be deleterious by generating superoxides and inflammatory mediators that could result in oxidative damage.10-13 Observational studies,14-20 although potentially confounded,21,22 have suggested that these complicated mechanisms, often collectively referred to as “storage lesion,” may adversely affect patient-important outcomes including infection, organ failure, hospital stay, and death.14-20
Prior evidence on the age of transfused RBCs has been dominated by uncontrolled observational studies that suggest better outcomes with fresher RBCs.23,24 However, a recently published 2015 Cochrane Systematic Review25 that focused exclusively on 16 randomized controlled trials was equivocal in their findings. This was a methodologically strong review, but because of lack of uniform definitions of “fresher” or “older” RBC storage and overlap in the distribution of the age of RBCs, the authors did not conduct any meta-analyses and noted that limitations in the evidence precluded definitive conclusions.
Subsequent to the Cochrane review,25 investigators have published 2 large trials26,27 that provided the impetus for our review. Here, we report on a systematic review and meta-analysis comparing the effects of fresher, newly stored RBCs vs older or standard-issue RBCs on mortality, adverse events, and infection.
Methods
Eligibility
We included randomized controlled trials enrolling patients of any age admitted to hospital and requiring a RBC transfusion comparing fresher vs older (or standard-issue) RBCs that reported mortality, adverse events, and/or post-transfusion infection. We did not exclude studies based on language or any other study characteristics.
Search
We accepted that the previously published Cochrane review25 had conducted a comprehensive search up to September 2014 and implemented their search strategy up to June 2015. Our electronic database search included MEDLINE, EMBASE, CINAHL (July 2014-June 2015), Cochrane Systematic Reviews (to June 2015), and the Cochrane Central Register of Controlled Trials (CENTRAL) (2014-2015) (see “Appendix” for details). We also searched PubMed for any very recent publications as of July 10 2015 and hand-searched reference lists of retrieved studies.
Study selection and data extraction
Two reviewers independently screened titles and abstracts in duplicate, obtained full texts of articles that either reviewer considered potentially eligible, and then determined eligibility from the full texts.
From the eligible studies, we abstracted data including single- or multicenter status, country, participant baseline characteristics, age of transfused RBCs, sample size, and outcome events. We also collected RBC characteristics—allogenic vs autologous, irradiated blood, whole blood, and leuko-reduced vs non–leuko-reduced RBCs—and the method of blood processing (eg, buffy coat, platelet-rich plasma).
Outcomes
We hypothesized that transfusion of fresher vs older RBCs would result in lower mortality, fewer adverse events, and a lower risk of infection. We used definitions from the primary studies for each outcome. Post-transfusion infections were defined broadly as any nosocomial infection requiring treatment after receiving the transfusion.
Risk of bias assessment
We addressed risk of bias using a modified Cochrane Risk of Bias Tool,28 which includes domains of random sequence generation, allocation concealment, blinding of participants and staff/lead researchers, blinding of outcome adjudicators, assessors, incomplete outcome data, selective outcome reporting, and early discontinuation. Response options were “yes,” “probably yes” (low risk of bias), “probably no,” and “no” (high risk of bias).29 We judged a study to be high risk of bias if the responses were “no” or “probably no” for the domains sequence generation, allocation concealment, blinding, or stopping early for benefit.
Reviewers independently made all eligibility, data abstraction, and risk of bias decisions and resolved disagreement through discussion and with third-party adjudication when necessary.
Rating quality of evidence
Statistical analyses
We assessed agreement for eligibility and risk of bias using chance-corrected κ.32 All other statistical analyses were conducted utilizing the Review Manager33 (RevMan) version 5.3.2. We calculated the pooled risk ratios of dichotomous outcomes using Mantel-Haenszel random-effects models and report pooled estimates and the corresponding 95% confidence intervals (CIs). We evaluate heterogeneity statistics using the Cochrane Q χ2 test and I2.34
Our primary analysis included only patients for whom outcome data were available (complete case) and counted patients in the groups to which they were randomized. For any clear differences in outcome between intervention and control groups, we planned to conduct sensitivity analyses using an approach in which we assumed worst plausible case results for patients in intervention groups with missing data.35 When outcomes were reported at different time points, we used data from the longest follow-up (eg, the trials for the mortality outcome).
Explanations of heterogeneity
To explain heterogeneity, we hypothesized that the following would be associated with a larger difference in favor of fresher RBCs: high- vs low-risk of bias; neonates/children vs adults; shorter storage of fresher RBCs (≤7 days vs 8-21 days for fresher RBCs) and longer storage of standard-issue RBCs (22-34 days vs 35-42 days); liberal vs restrictive transfusion strategy; and leukocyte-reduced RBCs vs non–leukocyte reduced RBCs. We conducted subgroup analysis only if I2 was > 0.
Results
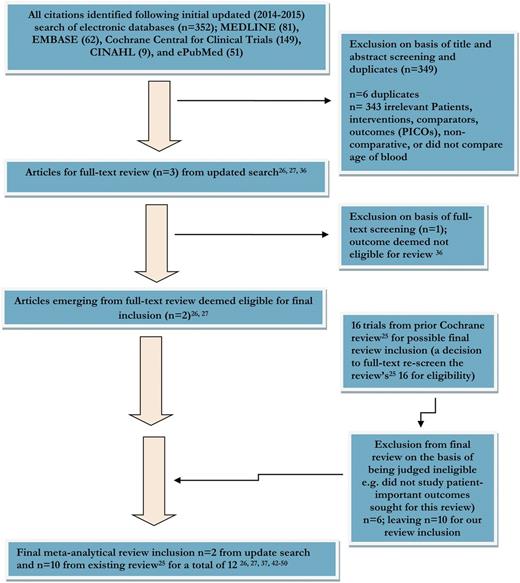
Of the 352 citations identified in our updated search, 2 proved eligible (Figure 1).26,27 Of the 3 articles that underwent full text review, one36 did not report the patient-important outcomes we sought. Of the 16 trials included in the recent Cochrane review,25 we judged 6 to be ineligible36-41 because they did not report the outcomes examined in this review, leaving a total of 12 eligible studies.26,27,37,42-50
Flow diagram of summary of evidence searching and final RCT selection.
Agreement (κ) for the title and abstract screening was 0.73, and for the completed full-text screening 0.71. Inter-rater agreement on individual domains of the risk of bias tool ranged from 0.51 to 0.69.
Table 1 presents trial characteristics. The trials included 5229 participants (1749 to 243027 ); 2451 patients were assigned to the fresher RBC arms and 2778 patients to the older RBC, or standard, arms. Six reports were feasibility trials intended to inform larger studies.37,42,44,45,47,49 Studies were conducted in Canada (3), USA (5), Brazil (1), Uganda (1), Australia (1), and European nations (with Canadian partnership) (1).
Transfused blood method of processing
No trial included information on whether buffy-coat or plasma-rich protein or other processing methods were used. Ten trials (83.0%) included information on leukoreduced status, 6 (50%) on irradiation status, and 6 (50%) on storage medium. Allogenic blood was used exclusively in 11 of 12 studies; one report49 did not specify.
Participant profile
Of the 12 eligible trials,26,27,37,42-50 3 enrolled neonates/infants with very low birth weight43,46,50 and 1 included patients 12 years and older26 ; the remainder enrolled adults. One trial was restricted to adults with malaria-associated anemia.45 Most trials enrolled patients experiencing acute critical illness and/or those with surgical hemorrhage.
Treatment and control arm interventions
Table 1 describes the interventions for included studies,26,27,37,42-50 and the definition of fresher RBCs vs older RBCs varies by study to the extent reported. Six trials compared fresher RBCs with older/longer storage RBCs,26,37,44-46,49 and 6 compared fresher RBCs with standard-issue or usual practice RBCs.27,42,43,47,48,50 For this report, however, older and standard-issue RBCs were considered the same because in both cases the oldest unit of RBCs in the inventory is issued for transfusion. Table 1 provides the mean (and standard deviation)/median (and interquartile range) ages (a reporting of age ranges) of transfused RBCs in both intervention arms.
Risk of bias
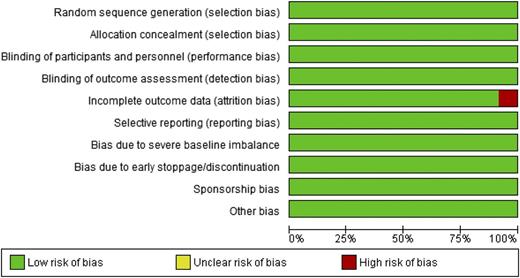
Studies were generally at low risk of bias (Figure 2).
Risk of bias assessment for included studies (domain risk percentages).
Outcomes
Mortality.
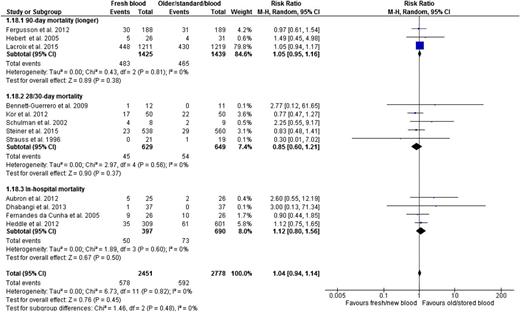
The risk of death, presented in all 12 trials, was similar in the fresher and older RBC groups (RR, 1.04; 95% CI, 0.94-1.14; P = .45; heterogeneity P = .82; I2 0%, moderate certainty in evidence) (Figure 3 and Table 2). We report mortality at longest follow-up for all trials and as shown, results were similar irrespective of maximum length of follow-up (interaction P = .48; Figure 3).
Fresh vs older blood outcome mortality by specific duration of follow-up (based on the longest duration of follow-up per trial).
Fresh vs older blood outcome mortality by specific duration of follow-up (based on the longest duration of follow-up per trial).
Adverse events.
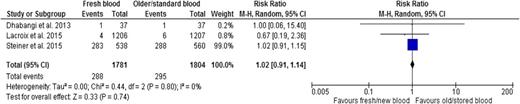
Three trials26,27,45 reported transfusion-related adverse events, which included acute transfusion reactions, seizures, acute cardiac events, hepatobiliary events, and renal and urinary events. Results indicated no difference in adverse events associated with age of RBCs (RR, 1.02; 95% CI, 0.91-1.14; P = .74; heterogeneity P = .80; I2 0%, low certainty in evidence) (Figure 4 and Table 2).
Infection.
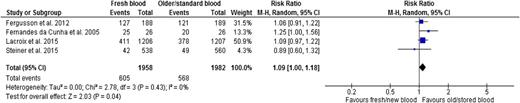
Four trials reported nosocomial infections, two in neonates,43,46 one in cardiac surgery adult patients,26 and one in critically ill adults.27 One reported microbiologically confirmed infection,43 one clinical sepsis in neonates,46 and two reported clinically diagnosed infections.26,27 There was a 9% relative increase in the risk in patients who received fresher RBCs (RR, 1.09; 95% CI, 1.00-1.18; P = .04; heterogeneity P = .43; I2 0%, low certainty in evidence) (Figure 5 and Table 2).
Sensitivity analysis
Because very few patients were lost to follow-up (range, 1.0%-3.2%), worst plausible assumptions35 of patients lost to follow-up did not materially change the results for any outcome.
Subgroup analysis
We did not conduct subgroup analyses because the heterogeneity was easily explained by chance (I2 = 0% for all analyses).
GRADE evidence profile
The accumulated evidence provides moderate certainty evidence for death and low certainty for adverse events and infections (Table 2). Although the total number of trial participants and the number of deaths is large, the confidence interval around mortality and adverse events includes benefit that most would consider important, and an increase in infection that most would consider important. Thus, for each outcome, we rated down for imprecision.
Discussion
Main findings
Evidence from 12 randomized controlled trials26,27,37,42-50 that included patients with varying ages and experiencing a range of medical and surgical conditions found no benefit when fresher RBCs were transfused as opposed to older/standard-issue RBCs for either mortality (Figure 3 and Table 2) or adverse events (Figure 4 and Table 2). Results suggested that, if anything, fresher RBCs might lead to an increase rather than a decrease in nosocomial infections (Figure 5 and Table 2). Confidence intervals were sufficiently wide that for each outcome we rated down certainty of evidence for imprecision.
Strengths and concerns/limitations
Strengths of our study include explicit eligibility criteria, a comprehensive search built upon a prior review,25 and reproducible duplicate assessment of eligibility, and risk of bias. We rated the certainty (or confidence) of evidence using the GRADE approach,30,31 highlighting the moderate certainty evidence for the impact of fresher vs older RBC transfusion on mortality, and the low certainty for adverse events and infections.
There are several concerns/limitations. Concerns about variability include not only those of patient group but also blood processing methods and definitions of fresher and older blood. The primary limitation of our study is the variability across trials in a number of characteristics, and associated concerns about the appropriateness of a pooled estimate given this variability. One source of variability is the definition of fresher vs older RBCs. Researchers used various cut-points to define fresh vs older or stored blood and also used variable measures to report on blood age (eg, mean or median, etc).
A second source of variability is the method of blood processing, as well as the storage solutions, leukocyte reduction (though the majority were reported as leuko-depleted), and irradiation. Manipulations that increase RBC fragility, such as irradiation and washing, may exacerbate the risk of prolonged storage through the mechanism of increased hemolysis and free iron.51 Hemolysis and free iron have been associated with multiple complications of transfusions and diseases such as sickle cell anemia.52,53 Authors did not, however, report these aspects of blood processing consistently or completely enough to allow for further analyses.
A third source of variability is the different populations, which included neonates and the pediatric and adult populations. Thus, one might raise concerns about combining results across studies given the variability in the age of fresher and older blood and differences in age between fresher and older blood, the variability in blood processing procedures, and the variability in patient characteristics and study methods. It is possible that the impact of fresher or older blood on mortality and other outcomes might differ across these factors. Conversely, it is possible that across these variations, the impact of fresher vs older blood is similar. The consistency of results across studies suggests that, in this instance, this is likely to be the case.
Another limitation is the sample size: although the total sample size and number of events are large, depending on one’s perspective, the data remain consistent with small but possibly important benefits of fresher RBCs on death rates. Using the median death rate of 12% in the old blood arms of the eligible trials, and the boundary of the confidence interval most favoring fresh blood, the largest benefit of fresh blood consistent with the results would be a reduction in mortality of 7 per 1000. Some might consider such a small mortality benefit not worth the economic and logistic burden of using exclusively fresh blood; others might disagree. Similarly, for infections, although an 8.6% absolute increase in infections with fresh blood (the upper boundary of the confidence interval) would be of great concern, an increase in infections of 1 per 1000 (the lower boundary of the confidence) would probably not. The backdrop is that RBC transfusion is one of the most common medical treatments, with approximately 85 million RBC units transfused annually worldwide54 ; hence, the impact of even a small risk could have significant patient impact globally.
Some suggest that there is increased chance of receiving older RBCs the more units of RBCs transfused.55,56 There may be a dose-response association operating in that in line with the storage lesion,14-20 and that although one unit of older RBCs would not be sufficient to cause adverse outcomes, transfusion of a number of units might.55,56 This is particularly relevant in regard to the safety of massive transfusions of older RBCs to infants and small children. These individuals might receive very large doses of older RBCs from a single unit of blood, and that unit may be particularly old (35-42 days). For example, observational data have suggested that infection rates are strikingly higher in infants and children undergoing cardiac surgery who receive washed transfusions of older RBCs (25-38 days).57 Researchers found that washing the RBCs that were oldest (eg, >29 days) was related to an increase in morbidity when compared with unwashed RBCs.57 An individual patient meta-analysis from studies that enrolled infants and small children who received very large volumes of washed RBCs may be a feasible approach to explore these issues.
Relation to prior work
Prior observational cohort studies, reviews, and meta-analyses based on cohort studies suggested that fresher vs older transfused RBCs reduced the risk of death and post-transfusion complications.19,23,58-61 Observational studies are, however, susceptible to prognostic differences between groups that could explain the apparent effect of RBC age. Thus, we relied exclusively on randomized trials to address the study question.
Our review results are largely consistent with those of a recent Cochrane review25 of randomized trials of the use of fresher vs older or stored RBCs for transfusion. Researchers reported no clear difference in mortality between fresher RBCs when compared with older RBCs.25 The prior Cochrane review being methodologically strong, did not, however, include the recently published large clinical trials,26,27 conduct meta-analysis, or use the GRADE approach; and the more recent trials26,27 substantially increased certainty in estimates of effect.
Ongoing trials
Several larger sample-sized international trials are ongoing that could address the limitations we have raised in our review. The methodologic quality of recently published trials26,27 and upcoming ones are needed to address the question of blood age on clinically significant outcomes. An ongoing Canadian multicenter trial (INFORM) of note in persons requiring a RBC transfusion seeks to determine the effect on in-hospital death rates of transfusing the freshest available RBCs compared with standard-issue RBCs.62 In addition, several trials (recruiting and not yet recruiting) are registered in the ClinicalTrials.gov regulatory database63 and worth flagging (eg, NCT01638416/examining whether patients who receive fresher RBCs do better than patients who receive standard issue RBCs; NCT01976234/examining transfusion of RBCs and the association with postoperative infections; NCT02393508/examining the impact of RBC age in patients receiving chronic blood transfusions in the outpatient setting) that, on completion, would help clarify our findings.
Conclusions
Our systematic review and meta-analysis provided no support for blood transfusion services implementing limits, or instituting preferential utilization, of RBC units that are fresh or stored for shorter periods. The consistent results across studies suggest that the impact of blood age does not differ across patient groups, nor that very fresh vs fresh RBCs, or old vs very old red RBCs, differ in their effects. Large ongoing studies may, however, challenge these results. It is more likely that they will show consistent results that further narrow the confidence intervals around key outcomes—and particularly mortality—and establish definitively that there is no need to change blood transfusion practices to ensure the use of younger or the freshest RBCs.
Acknowledgments
Ms Supriya Rave of Canada assisted in the title and abstract as well as full-text screening phases. Interpretations and conclusions drawn are not to be ascribed to her in any manner.
Authorship
Contribution: P.E.A. is the lead researcher on this research project and all coauthors have contributed in various means to the design, conduct and analysis, and writing/editing of the manuscript. Their contribution in terms of extent is indicated by the position in authorship. G.H.G. is the lead scientist with strong content guidance emerging from N.B. and N.M.H.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul Elias Alexander, Health Research Methods, Health Sciences Building, Department of Clinical Epidemiology and Biostatistics, McMaster University, 1280 Main St, West Hamilton, ON, L8N 3Z5, Canada; e-mail: elias98_99@yahoo.com.
Appendix: examples of the MEDLINE and EMBASE search built on the Cochrane25 search strategy, then limited to randomized controlled evidence
MEDLINE (Ovid SP)
ERYTHROCYTE TRANSFUSION/
((blood or erythrocyte* or red cell* or red blood cell* or RBC*) adj1 (transfus* or infus* or retransfus*)).ti,ab.
BLOOD TRANSFUSION/ or BLOOD COMPONENT TRANSFUSION/
ERYTHROCYTES/
(red cell* or red blood cell* or erythrocyte* or RBC* or whole blood).tw.
4 or 5
3 and 6
(RBC* or red cell* or red blood cell* or erythrocyte* or whole blood).ti.
1 or 2 or 7 or 8
exp Blood Preservation/
*Time Factors/
(age or aged or aging or fresh* or old or older or oldest or new or newer or newest or young or younger or youngest or store* or storage or storing or preserv*).ti.
10 or 11 or 12
9 and 13
((red cell* or red blood cell* or erythrocyte* or RBC* or blood or transfus*) adj5 (age or aged or aging or fresh* or old or older or oldest or new or newer or newest or young or younger or youngest or store* or storage or storing or preserv*)).ti.
((red cell* or red blood cell* or erythrocyte* or RBC* or whole blood) adj3 (store* or storage or storing or preserv* or fresh* or old or older or oldest or new or newer or newest or young or younger or youngest)).ab.
14 or 15 or 16
EMBASE (Ovid SP)
ERYTHROCYTE TRANSFUSION/
((blood or erythrocyte* or red cell* or red blood cell* or RBC*) adj1 (transfus* or infus* or retransfus*)).ti,ab.
BLOOD TRANSFUSION/ or BLOOD COMPONENT THERAPY/
ERYTHROCYTE/ or ERYTHROCYTE CONCENTRATE/
(red cell* or red blood cell* or erythrocyte* or RBC* or whole blood).tw.
4 or 5
3 and 6
(RBC* or red cell* or red blood cell* or erythrocyte* or whole blood).ti.
1 or 2 or 7 or 8
BLOOD STORAGE/
ERYTHROCYTE PRESERVATION/
STORAGE TIME/
*TIME/
(age or aged or aging or fresh* or old or older or oldest or new or newer or newest or young or younger or youngest or store* or storage or storing or preserv*).ti.
10 or 11 or 12 or 13 or 14
9 and 15
((red cell* or red blood cell* or erythrocyte* or RBC* or blood or transfus*) adj5 (age or aged or aging or fresh* or old or older or oldest or new or newer or newest or young or younger or youngest or store* or storage or storing or preserv*)).ti.
((red cell* or red blood cell* or erythrocyte* or RBC* or whole blood) adj3 (store* or storage or storing or preserv* or fresh* or old or older or oldest or new or newer or newest or young or younger or youngest)).ab.
16 or 17 or 18