Abstract
Chimeric antigen receptor T cell (CART) therapy has developed as a powerful and potentially curative therapy in hematological malignancies over the last few years. CD19 directed CART cells have resulted in impressive complete response rates of ~90% in acute lymphoblastic leukemia that are durable for the majority of patients. However, the overall response rates in other malignancies such as chronic lymphocytic leukemia are around 50%. This could be partially related to CART exhaustion and dysfunction induced by leukemia cells. In this study, we aim to evaluate the role of inhibitory receptors/pathways in inducing CART cell dysfunction and exhaustion in hematological malignancies.
As a tumor model, we used an acute myeloid leukemia (AML) cell line (MOLM14) and primary AML samples and treated them with CD33 or CD123 directed CART cells (second generation CARs using 4-1BB and CD3z signaling domains and a lentiviral vector).
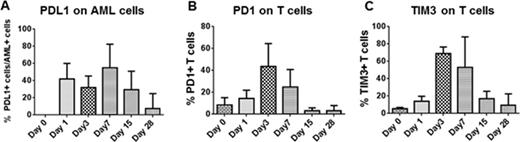
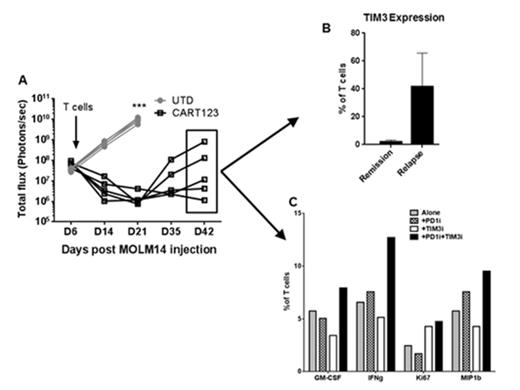
Incubation of primary AML samples or MOLM14 cell line with CD33 or CD123 directed CARTs resulted in a significant up-regulation of PD-L1 on tumor cells after 24 hours of incubation (0% on day 0 vs 80% on day 1, P <0.001), and up-regulation of PD-1 and TIM-3 on T cells 3-7 days post co-culture (8% of T cells expressed PD-1 on day 0 vs 43% on day 3, P=0.03 and 13% of T cells expressed TIM-3 on day 0 vs 71% on day 3, p=0.001, Figure 1). For in vivo experiments, we used NSG (NOD-SCID-g-/-) mice and engrafted them with the MOLM14 cell line. Treatment of these AML xenografts with suboptimal doses of CD33 or CD123 CARTs resulted in initial anti-tumor responses, followed by disease relapses in 40-60% of the mice (Figure 2A). T cells were isolated from the bone marrow of these mice and analyzed for differential expression of inhibitory receptors. There was a significantly increased up-regulation of TIM-3 receptors on T cells isolated from mice with relapsed disease compared with T cells isolated from mice in remission after CART cell therapy (Figure 2B).
Next, we investigated the role of adding checkpoint blockade to improve T cell function ex vivo after CART cell therapy. Marrows of mice that relapsed after CART cell therapy contained both residual CART cells and leukemia and were used to model the administration of checkpoint blockade in the setting of CART cell exhaustion. Cells were cultured with PD-1 or TIM-3 blocking antibodies or the combination of both (10 ug/ml for 72 hours). CART cell effector functions such as cytokine production and Ki-67 proliferation marker improved in the presence of checkpoint antagonists especially when both PD-1 and TIM-3 blocking antibodies were combined (figure 2C).
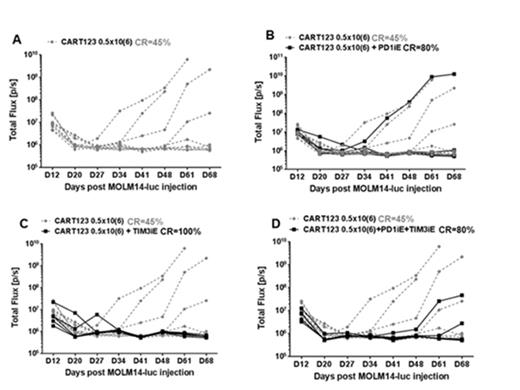
Finally, we tested the combination of checkpoint blockade with CARTs in AML xenografts. In this approach, we treated MOLM14 xenografts with suboptimal doses of CD33 or CD123 directed CARTs or with control untransduced T cells (UTD), with or without checkpoint blocking antibodies. NSG mice bearing MOLM14 AML xenografts were established. Engraftment was confirmed by bioluminescent imaging. The tumor bearing mice were then treated with suboptimal doses (0.25-0.5 x106 total T cells I.V) of CD33 or CD123 directed CARTs or with control untransduced T cells (UTD). Mice also received PD-1 blockade, TIM-3 blockade or the combination of both on days 3, 6, 9 and 12 post T cell therapy, with the rationale for early checkpoint blockade being based on our in vitro observations of early upregulation of inhibitory ligands on AML after exposure to CART cells. Mice were then followed with serial imaging to assess disease burden. The addition of checkpoint antagonists to untransduced T cells, in particular anti-TIM3, did not lead to an anti-leukemic effect. However, the addition of PD-1 or TIM-3 blockade to CART cell therapy resulted in a synergistic anti-tumor activity as shown in Figure 3. The durable complete response rate was: 45% for treatment with CART123 alone, 80% for treatment with CART123+PD-1 blockade, 100% for treatment with CART123+TIM-3 blockade, and 80% for treatment with CART123+ both PD-1 and TIM-3 blockade).
Our preclinical results indicate that PD-1 and TIM-3 pathways are involved in CART exhaustion and dysfunction in AML. Combination of checkpoint inhibitors with CART cells may lead to enhanced efficacy in AML and other hematological malignancies. Current studies are investigating mechanisms of synergy and the role of these combinations in other hematological malignancies.
Kenderian:Novartis: Patents & Royalties, Research Funding. Ruella:Novartis: Patents & Royalties, Research Funding. Porter:Novartis: Patents & Royalties, Research Funding. June:University of Pennsylvania: Patents & Royalties: financial interests due to intellectual property and patents in the field of cell and gene therapy. Conflicts of interest are managed in accordance with University of Pennsylvania policy and oversight; Novartis: Research Funding. Gill:Novartis: Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.