Abstract
Introduction:
Biologic drugs are high-cost technologies that represent a challenge to the economic management of healthcare systems. Biosimilar drugs (BSD) are designed to be highly similar, in terms of safety, purity and efficacy to an existing licensed reference biologic drug (RBD). Furthermore, the use of BSD can reduce up to 35% of the cost of treatment compared to RBD, so the approval of BSD worldwide will strongly influence prescriptions, particularly in emerging markets. Previous studies have identified challenges regarding BSD prescription and patient recruitment in developing countries. Our goal was to identify potential barriers to patient accrual during biosimilar clinical trials (CT) in Brazil and propose solutions to enhance the process.
Methods: A panel of specialists comprising investigators and site coordinators from 25 research sites in Brazil was invited to discuss recruitment barriers and related challenges in biosimilar trials. Participants were required to debate three open-ended questions. First: considering the biosimilar trial on rituximab, which are the main barriers/challenges to patient recruitment? Second: which aspects were determinant to an unsuccessful recruitment and why. Third: do the previous conclusions express my personal belief regarding the causes of the unsuccessful recruitment? Through a series of dynamic activities, problems were identified and collective solutions were proposed.
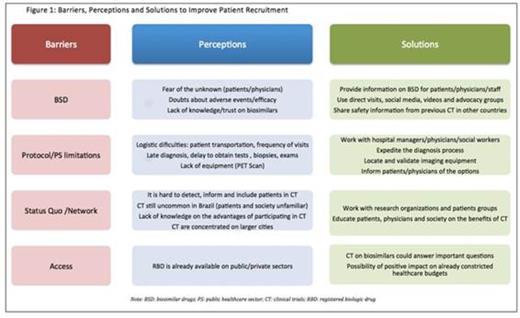
Results: A group of 16 investigators, 23 clinical trial coordinators and 14 members of the sponsors' staff participated in the meeting. Investigators had a mean experience of 20 years of clinical practice and 15 years on clinical trial conduction. Criteria for unsuccessful recruitment and investigators' beliefs regarding the causes of low recruitment were consistent among the answers. Barriers, perceptions and possible solutions to improve patient recruitment in BSD trials are depicted on Figure 1. The main barriers for recruitment were distributed among different domains, including the biosimilar drug itself, limitations on the public healthcare system (PS) and established perceptions (status quo), among others. According to the panel the main issues are lack of information regarding BSD among patients and physicians and the negative attitude toward CT participation among patients. The lack of physicians' and patients' awareness regarding biosimilar development pathways was emphasized by all the panelists. Also, logistics played and important role in limiting patients' participation.
Conclusions: The lack of information among patients and investigators was the main challenge regarding accrual in BSD trials, leading to negative attitudes towards CT in general by both patients and physicians. Providing information on BSD and CT to all players, as well as to society in general in fundamental in enhancing patient accrual and the comprehension on the benefits of participating in clinical studies.
Castilho:Libbs Farmacêutica: Employment. Almeida:Libbs Farmacêutica: Employment. Miyashiro:Libbs Farmacêutica: Employment.
Author notes
Asterisk with author names denotes non-ASH members.