Abstract
Background: The addition of a Tyrosine Kinase Inhibitor (TKI) to induction chemotherapy has improved the outcome of patients with Philadelphia chromosome positive (Ph+) acute lymphoblastic leukemia (ALL). However, the treatment related mortality and morbidity of intensive treatment increases with age. The use of a TKI alone for induction is less toxic and yields CR rates comparable to combined therapy. Eligibility for post remission hematopoietic stem cell transplantation is less likely to be compromised with TKI induction. We present a retrospective review of patients with Ph+ ALL treated at our institution with dasatinib and prednisone induction who subsequently underwent allogeneic hematopoietic stem cell transplant (allo-HSCT) as post remission therapy.
Methods: We retrospectively identified 15 patients with Ph+ ALL treated at our institution between February 2012 and June 2015. Patients received induction therapy with dasatinib at 100 mg or 140mg daily till complete hematological response. Prednisone 60 mg/m2/day (capped at 120 mg daily) was administered until day 24 and then tapered and stopped at day 32. Intrathecal chemotherapy with MTX and Ara-C were administered twice during the induction period. Dasatinib dose reduction/discontinuation was permitted for non-hematological toxicity. Patients who achieved remission proceeded to allo-HSCT if a suitable HLA-matched donor was available. Patients who did not have a suitable HLA matched donor received TKI + POMP maintenance. We calculated CHR, CCyR, disease-free survival (DFS) and overall survival (OS).
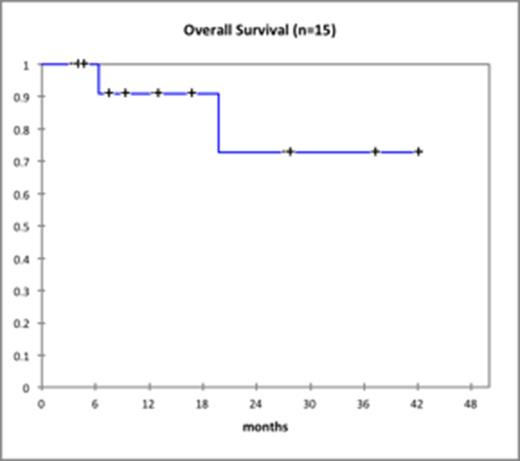
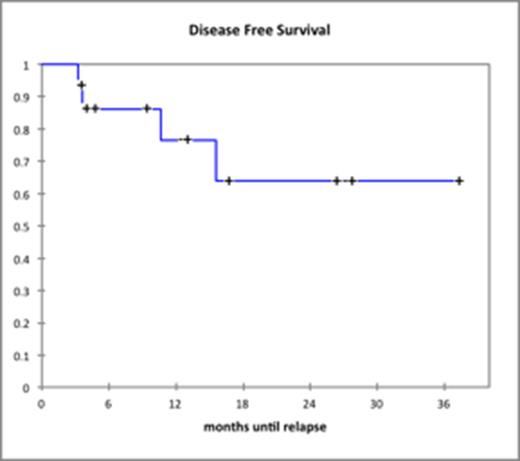
Results: The median age of patients treated with dasatinib plus prednisone was 62 years (range: 19-73). Baseline patient and disease characteristics are summarized in Table 1. Median WBC count was 22.5 x 109/L. Fourteen of 15 patients treated with dasatinib achieved a CHR (93.3%), 1 patient did not undergo a bone marrow biopsy but had normal blood counts. Median time to CHR was 42 days (range: 22-69). CCYR was obtained in 11 patients (73%) and MMR was achieved in 5 patients (33%). No patient died during induction therapy. The 14 patients who were in CHR after induction, underwent allo-HSCT (n=7), are being evaluated for allo-HSCT (n=3), were unable to undergo allo-HSCT due to a high comorbidity index and/or lack of a suitable donor (n=3) or were lost to follow-up (n=1). Of the 3 patients who were unable to undergo allo-HSCT, 2 patients continue on dasatinib maintenance and 1 patient takes ponatinib. Of 8 patients not yet transplanted 3 relapsed, while only 1 relapse was seen in 7 patients who underwent allo-HSCT. Median DFS was 315 days (range: 57-1061) and median OS was 354 days (range: 107-1082) corresponding Kaplan Meier curves for OS and DFS are shown below.
Conclusions: In our adult Ph+ ALL patients induction therapy with dasatinib and prednisone was effective and well tolerated. Patients achieving CHR were able to undergo allo-HSCT with curative intent. This strategy retrospectively appears equal or better than results with induction chemotherapy of conventional variety.
Patient characteristics
| Male sex, n (%) . | . | 5 (33.3) . | |
|---|---|---|---|
| Age | |||
| <20, n (%) | 1 (6.7) | ||
| 20-49, n (%) | 1 (6.7) | ||
| ³50, n (%) | 13 (86.6) | ||
| Median (range) | 62 (19-73) | ||
| Median follow-up in months (range) | 11.7 (4.1-40) | ||
| Presenting WBC x 10 9/L | |||
| < 30, n (%) | 8 (53.3) | ||
| ³ 30, n (%) | 7 (46.7) | ||
| Median (range) | 22 (2.8-358.4) | ||
| Bcr-Abl type | |||
| p190, n (%) | 12 (80) | ||
| p210, n (%) | 2 (13.3) | ||
| P190 and p210, n (%) | 1 (6.7) | ||
| Bcr-Abl level (1 unknown)* | Mean (range) | 35.1 (1.8-194.4) | |
| Median time to CHR in days (1 unknown), (range) | 41.5 (22-69) | ||
| Induction dose of dasatinib | |||
| 70mg BID, n (%) | 1 (6.7) | ||
| 100mg daily, n (%) | 8 (53.3) | ||
| 140mg daily, n (%) | 6 (40) | ||
| CCyR after induction achieved, n (%) | 11 (73.3) | ||
| MMR achieved after induction, n (%) | 5 (33.3) | ||
| Dasatinib Dosing after Induction | |||
| None, n(%) | 1 (6.7) | ||
| 70mg BID, n(%) | 1 (6.7) | ||
| 100mg/day, n(%) | 12 (80) | ||
| 140mg/day, n(%) | 1 (6.7) | ||
| POMP + TKI post induction, n(%) | 4 (26.7) | ||
| Post remission therapy (3 being evaluated for transplant, 1 never achieved CHR, 1 lost to ff-up)+ | |||
| Transplant, n (%) | 7 (46.7) | ||
| Ponatinib, n (%) | 1 (6.7) | ||
| Dasatinib, n (%) | 1 (6.7) | ||
| HyperCVAD±, n (%) | 1 (6.7) | ||
| TKI maintenance after transplant, n (% of transplanted) | 3 (42.9) | ||
| M351T mutation, n (%) | 1 (6.7) | ||
| F317L mutation, n (%) | 1 (6.7) | ||
| Male sex, n (%) . | . | 5 (33.3) . | |
|---|---|---|---|
| Age | |||
| <20, n (%) | 1 (6.7) | ||
| 20-49, n (%) | 1 (6.7) | ||
| ³50, n (%) | 13 (86.6) | ||
| Median (range) | 62 (19-73) | ||
| Median follow-up in months (range) | 11.7 (4.1-40) | ||
| Presenting WBC x 10 9/L | |||
| < 30, n (%) | 8 (53.3) | ||
| ³ 30, n (%) | 7 (46.7) | ||
| Median (range) | 22 (2.8-358.4) | ||
| Bcr-Abl type | |||
| p190, n (%) | 12 (80) | ||
| p210, n (%) | 2 (13.3) | ||
| P190 and p210, n (%) | 1 (6.7) | ||
| Bcr-Abl level (1 unknown)* | Mean (range) | 35.1 (1.8-194.4) | |
| Median time to CHR in days (1 unknown), (range) | 41.5 (22-69) | ||
| Induction dose of dasatinib | |||
| 70mg BID, n (%) | 1 (6.7) | ||
| 100mg daily, n (%) | 8 (53.3) | ||
| 140mg daily, n (%) | 6 (40) | ||
| CCyR after induction achieved, n (%) | 11 (73.3) | ||
| MMR achieved after induction, n (%) | 5 (33.3) | ||
| Dasatinib Dosing after Induction | |||
| None, n(%) | 1 (6.7) | ||
| 70mg BID, n(%) | 1 (6.7) | ||
| 100mg/day, n(%) | 12 (80) | ||
| 140mg/day, n(%) | 1 (6.7) | ||
| POMP + TKI post induction, n(%) | 4 (26.7) | ||
| Post remission therapy (3 being evaluated for transplant, 1 never achieved CHR, 1 lost to ff-up)+ | |||
| Transplant, n (%) | 7 (46.7) | ||
| Ponatinib, n (%) | 1 (6.7) | ||
| Dasatinib, n (%) | 1 (6.7) | ||
| HyperCVAD±, n (%) | 1 (6.7) | ||
| TKI maintenance after transplant, n (% of transplanted) | 3 (42.9) | ||
| M351T mutation, n (%) | 1 (6.7) | ||
| F317L mutation, n (%) | 1 (6.7) | ||
Bcr-Abl detection by PCR with unit in ratio (international scale),
+poor performance status or high comorbidity index is the reason for no transplant,
±hyperCVAD initiated but not tolerated.
Fazal:Novartis: Honoraria, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Speakers Bureau; Ariad: Consultancy, Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau. Off Label Use: Dasatinib use for newly diagnosed Ph+ ALL.
Author notes
Asterisk with author names denotes non-ASH members.