Abstract
Introduction: Hepatic VOD, also known as Sinusoidal Obstruction Syndrome, is a potentially life threatening complication of hematopoietic stem cell transplantation (HSCT). Severe VOD (sVOD), defined as VOD with evidence of multi-organ dysfunction, leads to high morbidity and greater than 80% mortality (Coppell 2010) in HSCT patients. The purpose of this study was to develop an approach, in the absence of a specific diagnosis code, to identify VOD and sVOD in a hospital administrative database and to measure incidence among HSCT patients.
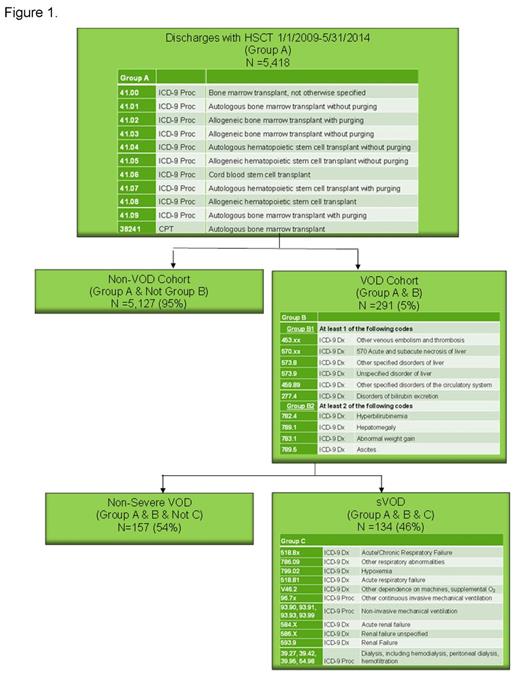
Methods: A retrospective cohort study was conducted using the Premier Healthcare Database, which contains more than 535 million patient encounters (inpatient and outpatient). The starting population was all patients with inpatient HSCT between January 1, 2009 and May 31, 2014 (n=5,418). Since there is currently no diagnosis code for VOD, we used clinical experience (CD, BN) to develop a coding algorithm to identify VOD within the database. In order to be categorized as having VOD, patients with HSCT had to have at least one code from the group B1, indicating hepatic or thromboembolic disease AND at least two codes from group B2, which is focused on VOD diagnosis criteria used in clinical practice (Figure 1). To more accurately identify VOD and exclude alternate etiologies of the liver abnormalities, patients with graft versus host disease (GVHD) (ICD-9 279.50) or TPN cholestasis (ICD-9 576.2 and TPN identified in charge master) were excluded. To qualify for sVOD, VOD patients also had to have at least one code from group C (Figure 1).
Results: This algorithm identified 291 VOD patients (5.4% of the HSCT patients) (Figure 1). Forty-six percent (n=134) of those VOD patients, or 2.5% of the total HSCT patients, had codes consistent with sVOD. The incidence of VOD among pediatrics (≤ 17 years old) was 6.4% and the incidence in adults (18+) was 5.3%. The mean age and standard deviation (SD) in the no-VOD and VOD groups was 50.6 (SD 18.4) and 50.6 (SD 19.2), respectively (p=0.97) and the mean age in the sVOD group was 47.7 (SD 21.4) (p=0.08 compared to no-VOD). Among non-VOD patients 8.6% were < 17 years, compared to 10.3% in all-VOD (p=0.647) and 14.9% in sVOD (p=0.096). Among allogeneic HSCT patients, 7.2% had VOD, including 3.6% with sVOD, while only 4.4% of patients with autologous HSCT had VOD, including 1.8% with sVOD.
Discussion: The incidence of VOD found in this study was lower than some literature estimates. Coppell et al., using a systematic literature review from 1979 through 2007, found an overall VOD incidence of 13.7%, with higher incidence in patients with allogeneic HSCT (12.9%) than in autologous (8.7%). However, this estimate includes older studies in a time when more intensive conditioning led to higher rates of VOD. Furthermore, our criteria were designed to identify patients with high certainty of VOD. In a more recent study in which 271 patients received reduced intensity conditioning for allogeneic HSCT, the cumulative incidences of VOD and sVOD were 8.9% and 1.5%, respectively (Tsirigotis 2014).
Limitations: This analysis is subject to limitations of administrative data. Since there is no diagnostic code for VOD, we used clinical judgment to sort through diagnosis and procedures codes that could be associated with complications of HSCT to find the most likely codes related to VOD and sVOD. The lower incidence in our analysis may have been the result of relatively restrictive methodology designed to identify VOD patients with a high degree of certainty based on diagnosis codes.
Conclusion: VOD is a potentially life threatening complication of HSCT and was found in 5% of inpatient HSCT procedures, half of which were deemed severe with multi organ dysfunction, in the 5-year period ending in mid-2014.
Dvorak:Jazz Pharmaceuticals: Consultancy. Nejadnik:Jazz Pharmaceuticals: Employment, Equity Ownership. Cao:Jazz Pharmaceuticals: Other: Premier Research Services received funding from Jazz Pharmaceuticals for data access, study design, and analysis; Premier Research Services: Employment. Lipkin:Jazz Pharmaceuticals: Other: Premier Research Services received funding from Jazz Pharmaceuticals for data access, study design, and analysis; Premier Research Services: Employment. Robinson:Jazz Pharmaceuticals: Other: Premier Research Services received funding from Jazz Pharmaceuticals for data access, study design, and analysis; Premier Research Services: Employment. Villa:Jazz Pharmaceuticals: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.