Abstract
The Hematopoietic Cell Transplant Co-morbidity Index (HCT-CI, Sorror et al 2005) was developed as a prognostic tool for overall survival (OS) and non-relapse mortality (NRM) in allogeneic hematopoietic cell transplant (HCT) patients. The prognostic significance of the score for patients with acute myeloid leukemia (AML) undergoing HCT has been demonstrated, however reports are conflicting. The purpose of this single-center study was to retrospectively investigate the prognostic impact of the individual component co-morbidities of the HCT-CI on the outcome of 418 patients that underwent HCT for AML at our center between 2000 and 2013.
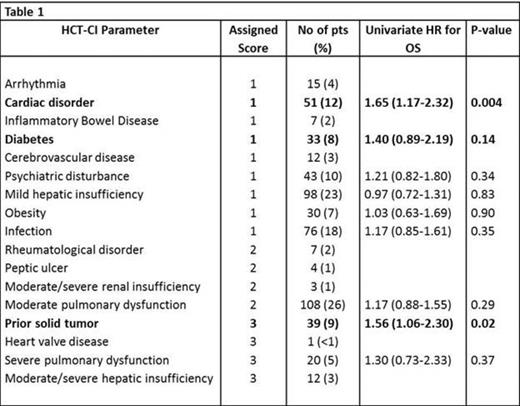
Patients underwent HCT in first (CR1, n=303) and second (CR2, n=115) complete remission. Median age at HCT was 50 years (range 18-71), 212 (51%) patients were female. Myeloablative conditioning (MAC) was used in 283 (68%) patients, reduced-intensity (RIC) in 135 (32%) patients. Donors were related for 236 (56%) patients, unrelated for 182 (44%) patients. Grafts were peripheral blood stem cells (PBSC) in 339 (81%) patients and bone marrow in 79 (19%) patients. Median follow-up of patients alive was 62 months (range 12-168). Cytogenetics at diagnosis were available for 84% of patients, of which 31 (7%) were favorable, 246 (59%) were intermediate and 74 (18%) were unfavorable risk (MRC classification). HCT-CI scores were grouped as 0 (n=109, 26%), 1-2 (n=157, 38%) and ≥3 (n=152, 36%). A total of 171 patients (41%) underwent HCT during the years 2000-2006 and 247 patients (59%) during the years 2007-2013. The observed frequency of the co-morbidities composing the HCT-CI is summarized in Table 1.
Univariate analysis for OS demonstrated the following significant variables: Age (HR=1.02, 95%CI=1.01-1.03, p=0.0002), CR status (HR=1.42 for CR2, 95%CI=1.08-1.87, P=0.01), donor type (HR=0.73 for related, 95%CI=0.57-0.94, p=0.02), HCT-CI group (overall p-value=0.004). For OS, univariate analysis of the impact of individual co-morbidities was performed for the components of the HCT-CI score that were observed in ≥5% of the patients (Table 1). All variables with a p-value ≤0.2 were introduced into the multivariable analysis (not including the HCT-CI itself), and these included cardiac disorder (CAD, CHF, MI or EF≤50%) (HR=1.65, 95%CI=1.17-2.32, p=0.004), prior solid tumor (HR=1.56, 95%CI=1.06-2.30, p=0.02) and diabetes (HR=1.40, 95%CI=0.89-2.19, p=0.14). In the multivariable analysis for OS, none of the aforementioned co-morbidities demonstrated independent prognostic relevance. For NRM, univariate analysis demonstrated cardiac disorder (HR=1.89, 95%CI=1.27-2.81, p=0.002), diabetes (HR=1.94, 95%CI=1.20-3.12, p=0.007) and moderate pulmonary (FEV1 and/or DLCO 66-80% or dyspnea on slight activity) (HR=1.31, 95%CI=0.93-1.84, p=0.12) to meet the significance criteria for inclusion in the multivariable analysis, which finally demonstrated diabetes (HR=2.17, 95%CI=1.31-3.60, p=0.003) and cardiac disorder (HR=1.78, 95%CI=1.15-2.76, p=0.01) to be independent predictors of NRM post-transplant.
In conclusion, among the pre-transplant co-morbidities included in the HCT-CI, diabetes and cardiac dysfunction are independent prognostic indicators for NRM but not for OS. Pulmonary dysfunction does not seem to negatively influence outcomes in this cohort of patients.
Kim:Novartis Pharmaceuticals: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.