Abstract
BACKGROUND
Hodgkin lymphoma (HL) incidence peaks in adolescence and young adulthood. The care of patients in this age range must address unique challenges as it spans the transition from pediatric to adult centers and treatment approaches vary considerably. The current standard of care for treatment of HL in adults is doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) with varying use of consolidation radiation therapy (RT). Multiple large trials in adults have demonstrated excellent long-term survival outcomes for both limited and advanced stage disease, with low rates of long-term toxicity. In British Columbia, Canada, ABVD was adopted for primary treatment of HL in the pediatric population in 2004. Pediatric, adolescent and young adult outcomes following this approach, in combination with reduced rates of RT, have not been reported. We report outcomes of a population-based cohort of children (age <18y) and young adults (18-25y at diagnosis) with HL who received a standardized ABVD regimen across adult and pediatric centers.
PATIENTS AND METHODS
The study cohort included 222 patients (n=58 < 18y; n=164 18-25y) with all histological subtypes of HL diagnosed from January 1, 2004 to July 1, 2013 who received ABVD as initial therapy. 87 patients had limited stage (IA, IB, IIA, bulk < 10 cm) and 135 had advanced stage disease (IA-IIA with bulk >10 cm and IIB-IVB). Limited stage patients received 2 cycles of ABVD followed by PET/CT assessment. Consolidation treatment consisted of involved-field radiation therapy (IFRT) to the area of original disease for incomplete responders (PET positive) or 2 further cycles of ABVD. Advanced stage patients were treated with 6 cycles of ABVD followed by repeat imaging. IFRT to areas of residual disease (PET positive and/or persistent mass by CT scan) was recommended for those patients with incomplete response.
RESULTS
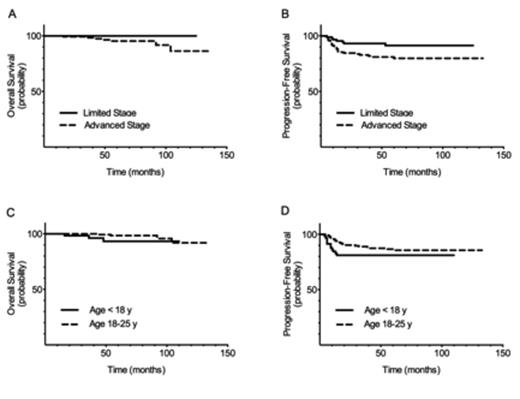
There were no significant differences in gender, histology, B symptoms or bulk between children (< 18y) and young adults (18-25y). The pediatric group had higher proportions of Ann Arbor stage III or IV disease (p =.002), possibly related to more frequent use of PET/CT scanning for staging (66% vs 10%, respectively, p= <.001), but the groups were similar following risk stratification into limited (36% vs 40%, respectively) and advanced (64% vs 60%, respectively) stage disease (p =.588).
The median follow-up was 64 months. More young adults achieved complete response at end of therapy (95% vs 83%; p =.004). Children were more likely to have primary progressive disease with 9 of 11 treatment failures (82%) occurring during treatment or within 6 months of completion. Patients aged 18-25y were more likely to have late treatment failures, with 41% of relapses occurring greater than 12 months after therapy completion (p =0.014). There were no toxicity-related deaths.
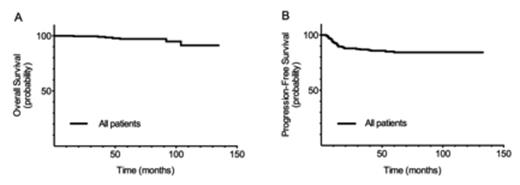
The 5 year overall survival (OS) and progression-free survival (PFS) for the entire cohort (n=222) were 97±1% and 84±3%, respectively. In limited stage disease, OS and PFS were 100% and 91±3%, while those with advanced stage had an OS of 95±2 % and PFS of 80±4%. There was no difference in OS (93±4% vs 98±1%, p =.120) or PFS (81±5% vs 85±3%, p =.222) between children and young adults. The rate of consolidative RT was 20% overall (24% in limited stage; 18% in advanced stage) with no difference between age groups (p =.295).
CONCLUSION
ABVD is an effective treatment for HL in the pediatric, adolescent and young adult populations. Radiation therapy can be omitted for the 75% of patients who achieve a complete response to chemotherapy while maintaining comparable 5 year overall survival outcomes, thus limiting the risk of radiation related long-term toxicities.
Overall Survival and Progression-Free Survival by Risk Group and Age Group
Connors:Roche: Research Funding; Seattle Genetics: Research Funding. Savage:Seattle Genetics: Honoraria, Speakers Bureau; BMS: Honoraria; Infinity: Honoraria; Roche: Other: Institutional research funding.
Author notes
Asterisk with author names denotes non-ASH members.