Abstract
Background
Spleen tyrosine kinase (SYK) is a nonreceptor cytoplasmic kinase that binds to phosphorylated immunoreceptor tyrosine-based activating motifs and mediates cellular proliferation and survival. SYK plays a key role in B-cell receptor signaling-driven tumorigenesis; thus, inhibition of SYK represents a viable therapeutic approach for various B-cell malignancies. Preclinical studies of TAK-659, an investigational, reversible SYK inhibitor, demonstrated inhibition of SYK activity and cell proliferation in vitro and dose-dependent tumor growth inhibition in vivo in lymphoma xenograft models. The primary objectives of this first-in-human phase 1 study (NCT02000934) are to determine the safety, tolerability, and maximum tolerated dose (MTD)/recommended phase 2 dose (RP2D) of TAK-659. Secondary objectives include evaluation of the preliminary antitumor activity and the pharmacokinetics (PK) of TAK-659.
Methods
Pts aged ≥18 yrs with advanced solid tumors or lymphoma, for which standard treatment was either not available or no longer effective, received oral TAK-659 daily (QD, 60-120 mg) in 28-d cycles. For MTD determination, dose escalation proceeded via a modified titration design based on dose-limiting toxicities (DLTs) or drug-related grade ≥2 adverse events (AEs) during cycle 1 (C1). Cell of origin classification data (activated B cell [ABC] vs germinal center B cell [GCB] subtype via immunohistochemistry) were collected from pts with diffuse large B-cell lymphoma (DLBCL). AEs were assessed per NCI-CTCAE v4.03. Blood samples for plasma PK assessments were collected pre-dose and at multiple times post-dose on d1 and d15 of C1. Response per RECIST v1.1 (solid tumors) and per IWG modified criteria (lymphoma) was assessed between d22 and d29 (pre-dose, d1 next cycle) of C2, C4, and C6, and then every 3 cycles.
Results
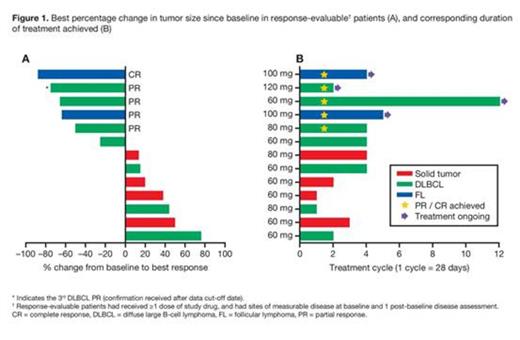
At data cut-off (June 15, 2015), 24 pts had been enrolled (14 solid tumor pts; 10 lymphoma pts) at 4 dose levels of TAK-659 (60, 80, 100, 120 mg). Overall, the median age was 58 years [range 43-80], 67% were male, and 58% had ≥4 prior lines of therapy. Of 10 lymphoma pts, 7 had DLBCL (5 GCB and 2 ABC subtypes) and 3 had follicular lymphoma (FL). Six pts remain on the study; 2 solid tumor and 4 lymphoma pts. The median number of TAK-659 cycles was 2 (range 1-4) for solid tumor pts and 4 (1-12) for lymphoma pts (Figure 1). C1 DLTs occurred in 5 pts; 1 pt at 60 mg (grade 3 asymptomatic aspartate aminotransferase [AST] elevation) and 4 pts at 120 mg (grade 3 and 4 asymptomatic lipase elevation, grade 3 mucositis, and grade 3 generalized edema). MTD determination is ongoing. All grade drug-related AEs occurred in 18 pts (75%) overall; the most common were fatigue (25%), elevated AST (21%), anemia (17%), and diarrhea (17%). Grade ≥3 drug-related AEs occurred in 8 pts (33%); anemia (1 pt at 60 mg, 2 pts at 120 mg), hypophosphatemia (2 pts at 80 mg), and increased lipase (2 pts at 120 mg) were seen in ≥1 pt. Four pts discontinued due to AEs (1 considered related to TAK-659, grade 3 pneumonia); 5 pts died on study (deaths were not considered related to TAK-659). Preliminary plasma PK of TAK-659 (n=24, 60-120 mg) showed rapid absorption (median Tmax 2 hrs), moderate variability in steady-state exposures (54% coefficient of variation for d15 dose-normalized AUCtau), mean peak-to-trough ratio of 4, and accumulation of 2.3-fold after repeated QD dosing for 15 d. Of 7 response-evaluable DLBCL pts, 2 pts (1 at 60 mg [GCB], 1 at 80 mg [ABC]) achieved partial responses (PRs), and a 3rd pt (at 120 mg [GCB]) achieved a PR post data cut-off (Figure 1). One GCB-DLBCL responder with multiple prior therapies including autologous hematopoietic stem cell transplantation is ongoing in C12. Two of 3 FL pts (both at 100 mg) were response-evaluable - 1 achieved a PR after 2 cycles (ongoing in C5) and 1 achieved a complete response after 2 cycles (ongoing in C4). Updated results will be presented at the meeting.
Conclusions
Oral TAK-659 60-100 mg QD appears to have an acceptable safety profile with an acceptable PK profile that supports continuous oral QD dosing. Early antitumor activity in DLBCL and FL pts is evident. Expansion cohorts are planned in pts with DLBCL and chronic lymphocytic leukemia. In addition, TAK-659 is also a potent FLT-3 inhibitor; a separate ongoing clinical trial is investigating the dual SYK and FLT-3 inhibitory activity of TAK-659 in relapsed/refractory acute myelogenous leukemia (NCT02323113).
Petrich:Gilead: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Spectrum Pharmaceuticals: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Research Funding. Off Label Use: This is a first-in-human phase 1 study of TAK-659, an investigational SYK inhibitor, in adult patients with advanced solid tumor or lymphoma malignancies.. Gordon:Northwestern University: Employment; Dr Leo I. Gordon: Patents & Royalties: Patent for gold nanoparticles pending. Infante:Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Research Funding. Madan:Celgene: Speakers Bureau; Onyx: Speakers Bureau. Stumpo:Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Zhang:Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Faucette:Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Shou:Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment.
Author notes
Asterisk with author names denotes non-ASH members.