Abstract
Introduction: Interferon-alpha (IFNα) has been successfully used to treat myeloproliferative neoplasms (MPN) for 30 years, although never officially registered for this indication. Studies with new pegylated interferons are underway to finally achieve IFNα registration for MPN.
Aims: The aim of this retrospective analysis was to investigate the long-term survival of polycythemia vera (PV) patients that received IFNα while being treated at the outpatient clinic of the Medical University of Vienna, a center that has been treating MPN with IFNα since 1984, and to compare it to patients that received best available therapy (BAT). All treatments were based on international recommendations and standardized center specific guidelines.
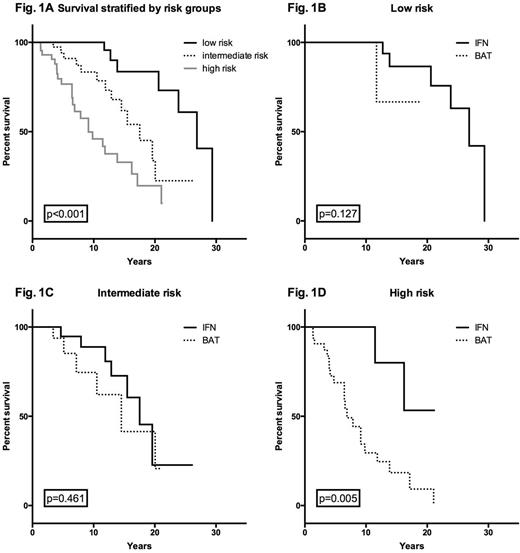
Methods: The medical histories of patients diagnosed with PV according to the PVSG- or WHO-criteria were investigated and information on the course of the disease and on cytoreductive treatment was collected. For a patient to be included in the analysis, IFNα (conventional or pegylated IFNα2a, -2b, or IFNα2c, or lymphoblastoid IFNα) had to be given for at least 365 days, or, in patients who were followed for more than 10 years, for at least 10% of the observation period after the diagnosis of PV. IFNα-patients who did not fulfill these criteria were not included. The IFNα-patients were compared to patients who received BAT consisting of other cytoreductive agents and/or phlebotomy. A portion of IFNα-patients also received other cytoreductive agents. The patients were then divided into three risk groups based on a previously published score based on age at diagnosis (<57, 57-66, ≥67), venous thromboembolism at baseline and leukocyte counts at diagnosis ≥15 G/L (Fig. 1A). Kaplan-Meier plots were used to compute overall, fibrosis- and AML-free survival.
Results: Of 129 patients, 67 received IFNα, 62 BAT (22 received no cytoreductive therapy). Median duration of IFNα treatment was 1981 days. Overall survival was generally better in patients treated with IFNα in all three risk groups. While the difference in the intermediate risk group did not reach significance (Fig. 1C), the survival benefit in the low risk group narrowly missed significance (p=0.127, Fig. 1B). More importantly, IFN-patients in the highest risk group lived significantly longer than patients receiving BAT (p=0.005, Fig. 1D). Median age in all three risk groups did not differ between the two groups, ruling out age as a possible confounder. Also, there were no other relevant differences between any of the groups. Although fibrosis-free survival differed in the low and intermediate risk group, no significant differences were observed. There was no benefit in leukemia-free survival in this analysis.
Discussion: This study supports the theory that IFNα can have a beneficial impact not only on symptoms and blood counts of PV-patients, but also on the long-term outcome in regard of overall survival, even in high risk patients. However, due to the retrospective nature of this study, the low case number in some subgroups and the lack of information on the fitness of the individual patients, our results can only serve as an impulse on future studies investigating the long-term outcome of IFNα-patients in MPN.
Off Label Use: Interferon-alpha, use in myeloproliferative neoplasms. Gisslinger:AOP ORPHAN: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen Cilag: Honoraria, Speakers Bureau; Geron: Consultancy; Sanofi Aventis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract