Abstract
Background: localized ocular adnexal mucosa-associated lymphoid tissue lymphoma (OAML) was effectively treated with frontline radiotherapy. however it is sometimes associated with radiation-related complications of ocular structures, as well as distant relapse or delayed relapse. Although there are no definitive optimal treatment approaches for these patients, we investigated the results in accordance with frontline treatment modalities for OAML.
Patients and methods: 159 patients with biopsy-confirmed primary OAML between 2007 and 2015 were analyzed retrospectively. All the patients were sorted by risk-stratification with Ann-Arbor staging and the tumor, node, metastasis (TNM) staging system, and treatment modalities according to anatomic location, prognosis factors, which were supported by ophthalmologist.
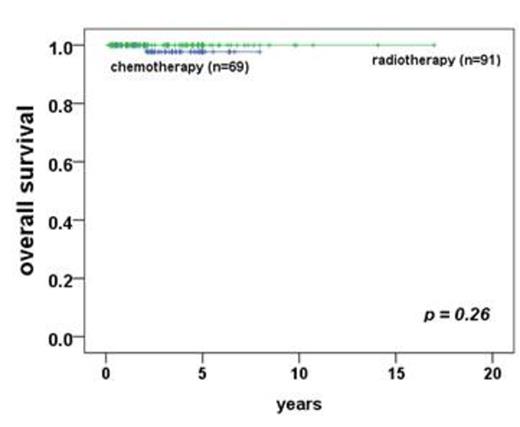
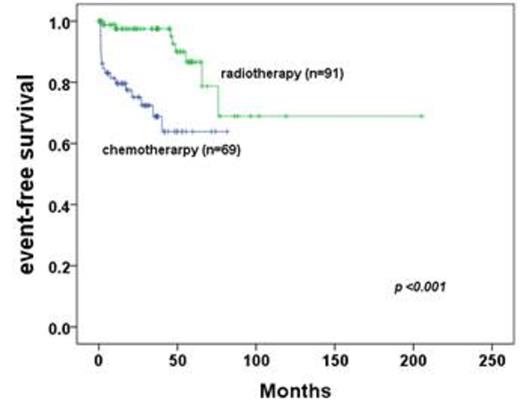
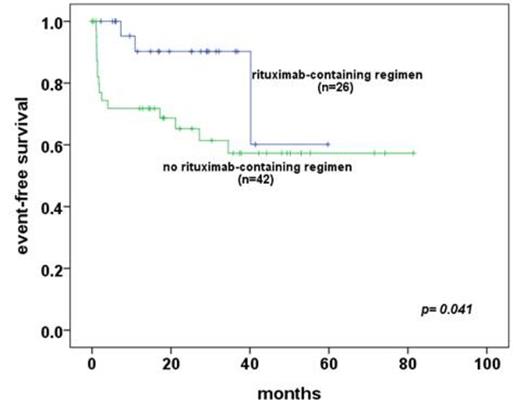
Results: first-line radiotherapy had overall survival(OS) of 100%, event-free survival(EFS) of 90%. However frontline-radiotherapy group has 100% of survival with 55% of moderate to severe dry eye syndrome, 37.4% of cataract incidence, 11% of cataract-related operations, and 1% (n=1) of radiation-related blindness. Although 5-year EFS is good in radiotherapy group (90% vs 64% in radiotherapy group vs chemotherapy, p<0.001), most of aggressive factors (young age at diagnosis, conjunctival lesions, Ann Arbor stage I, early T1N0M0 stage, and no distant extranodal metastasis including of bone marrow involvement) showed in radiotherapy group in comparison with chemotherapy group. In subgroup analysis of first-line chemotherapy, rituximab-containing group has more good response of event-free survival (p= 0.041) with tolerable hematologic toxicities compared to frontline radiotherapy group.
Conclusion: OAML with localized to conjunctiva alone was treated with frontline-radiotherapy and non-conjuctival lesions treated with chemotherapy. Radiotherapy as first-line is good local control-therapeutic option in patients with localized to conjunctiva alone, but management of radiation-associated complication is concerned troubles. In advanced OAML including of extra-conjunctival lesions, systemic chemotherapy is best options. rituximab-based regimen could be feasible to obtain good disease-free survival and response rate.
Patient's characteristics between frontline-chemotherapy and radiation group (n= 159)
| Factors . | Primary Chemotherapy group (n = 68) . | Primary Radiation group (n = 91) . | P-value . |
|---|---|---|---|
| Gender (male) | 37 (54%) | 28 (30.8%) | 0.003 |
| Age at diagnosis (median, year) | 50 | 43 | 0.003 |
| involvement of both eyes at diagnosis | 22 (32.4%) | 23 (25.3%) | 0.75 |
| Conjuctival lesion at diagnosis | 22 (32.4%) | 76 (83.5%) | <0.001 |
| Ki-67 on pathologic stain (median, %) | 5 | 4 | 0.545 |
| Ann-Arbor stage I | 23 (66.2%) | 3 (96.7%) | <0.001 |
| LDH at diagnosis (mg/L) | 355 | 344 | 0.216 |
| Patients no. with T1N0M0 | 21 (30.9%) | 78 (85.7%) | <0.001 |
| Bone marrow involvement | 7 (10.3%) | 0 (0%) | <0.001 |
| Patients no. with N1~N3 staging | 10 (14.7%) | 0 (0%) | <0.001 |
| Patients no. with T0N0M1~2 | 7 (10.3%) | 0 (0%) | <0.001 |
| Factors . | Primary Chemotherapy group (n = 68) . | Primary Radiation group (n = 91) . | P-value . |
|---|---|---|---|
| Gender (male) | 37 (54%) | 28 (30.8%) | 0.003 |
| Age at diagnosis (median, year) | 50 | 43 | 0.003 |
| involvement of both eyes at diagnosis | 22 (32.4%) | 23 (25.3%) | 0.75 |
| Conjuctival lesion at diagnosis | 22 (32.4%) | 76 (83.5%) | <0.001 |
| Ki-67 on pathologic stain (median, %) | 5 | 4 | 0.545 |
| Ann-Arbor stage I | 23 (66.2%) | 3 (96.7%) | <0.001 |
| LDH at diagnosis (mg/L) | 355 | 344 | 0.216 |
| Patients no. with T1N0M0 | 21 (30.9%) | 78 (85.7%) | <0.001 |
| Bone marrow involvement | 7 (10.3%) | 0 (0%) | <0.001 |
| Patients no. with N1~N3 staging | 10 (14.7%) | 0 (0%) | <0.001 |
| Patients no. with T0N0M1~2 | 7 (10.3%) | 0 (0%) | <0.001 |
Patients treated with frontline chemotherapy were classified in more aggressive or advanced stage which compare to patients treated with primary radiotherapy statistically.
Patient's characteristics between Rituximab containing group and non-rituximab in frontline chemotherapy subgroup (n=68)
| Factors . | Ritixumab-containing (n = 26) . | No Rituximab (n = 42) . | P-value . |
|---|---|---|---|
| Gender (male) | 0.942 | ||
| Age at diagnosis (median, year) | 52 | 49 | 0.411 |
| involvement of both eyes at diagnosis | 0.057 | ||
| Conjuctival lesion at diagnosis | 0.087 | ||
| Ki-67 on pathologic stain (median, %) | 4 | 3 | 0.540 |
| Ann-Arbor stage I | 0.532 | ||
| LDH at diagnosis (mg/L) | 353 | 356 | 0.782 |
| Patients no. with T1N0M0 | 0.585 | ||
| Patients no. with N1~N3 staging | 0.659 | ||
| Patients no. with T0N0M1~2 | 0.695 |
| Factors . | Ritixumab-containing (n = 26) . | No Rituximab (n = 42) . | P-value . |
|---|---|---|---|
| Gender (male) | 0.942 | ||
| Age at diagnosis (median, year) | 52 | 49 | 0.411 |
| involvement of both eyes at diagnosis | 0.057 | ||
| Conjuctival lesion at diagnosis | 0.087 | ||
| Ki-67 on pathologic stain (median, %) | 4 | 3 | 0.540 |
| Ann-Arbor stage I | 0.532 | ||
| LDH at diagnosis (mg/L) | 353 | 356 | 0.782 |
| Patients no. with T1N0M0 | 0.585 | ||
| Patients no. with N1~N3 staging | 0.659 | ||
| Patients no. with T0N0M1~2 | 0.695 |
There are statistically no differences between patients treated with Rituximab-containing regimen and non-rixuximab in frontline chemotherapy subgroup.
*event-free survival / overall survival between primary chemotherapy group and patients with radiotherapy * event: from partial response to progressive disease after completion of primary treatments, or death.
*event-free survival / overall survival between primary chemotherapy group and patients with radiotherapy * event: from partial response to progressive disease after completion of primary treatments, or death.
*event-free survival between patients with rituximab-containing regimen and without rituximab in subgroup analysis of frontline-chemotherapy group * event: from partial response to progressive disease after completion of primary treatments, or death
*event-free survival between patients with rituximab-containing regimen and without rituximab in subgroup analysis of frontline-chemotherapy group * event: from partial response to progressive disease after completion of primary treatments, or death
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.