Abstract
INTRODUCTION: Hemophagocytic lymphohistiocytosis (HLH) is a rare disorder caused by the pathologic activation of the immune system. In children, either a molecular diagnosis consistent with HLH or five out of the following eight criteria are considered necessary for a diagnosis of HLH (HLH-04 criteria): 1) fever; 2) splenomegaly; 3) cytopenia in two or more cell lines; 4) hypertriglyceridemia (≥265 mg/dL) or hypofibrinogenemia (≤150 mg/dL); 5) hemophagocytosis in the bone marrow, spleen, or lymph nodes; 6) hyperferritinemia (≥500 mcg/L); 7) impaired NK cell function; and 8) elevated soluble CD25 (sCD25). These criteria have been extrapolated to diagnose HLH in adults; however, it's unclear if these same criteria are applicable in the adult population.
METHODS: We reviewed the Mayo Clinic electronic medical record for all adult (≥18 years) hospitalized patients with an admission serum ferritin of ≥500 mcg/L from January 2012 through December 2014. Patients' charts were reviewed and those who met the HLH-04 criteria were considered to have HLH. For the remainder of the patients, the etiology for hyperferritinemia was determined based on chart review and discharge diagnoses. Logistic regression models were used to assess the ability of these values in predicting a diagnosis of HLH. The Mayo Clinic IRB approved this study.
RESULTS: We identified 1,329 patients with a serum ferritin ≥500 mcg/L. Of these, HLH was diagnosed in 28 (2.1%) patients (malignancy-associated HLH in 11 patients, infection-associated HLH in 4 patients, autoimmune-associated HLH in 7 patients, and idiopathic HLH in 6 patients). Table 1 describes the etiology of hyperferritinemia in the remaining 1,301 patients. In contrast to pediatric hospitalized patients (Allen, Pediatr Blood Cancer, 2008), adults are more likely to have malignancy (28.1% vs 7%; p<0.05); bacterial infection (21% vs. 13%; p=0.001); liver disease (9.9% vs. 2.7%; p<0.05); and cardiac disease (7.2% vs. 1.5%; p=0.0001) as the etiology of hyperferritinemia during their hospitalization.
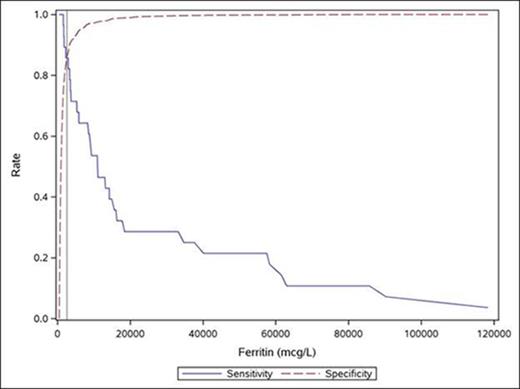
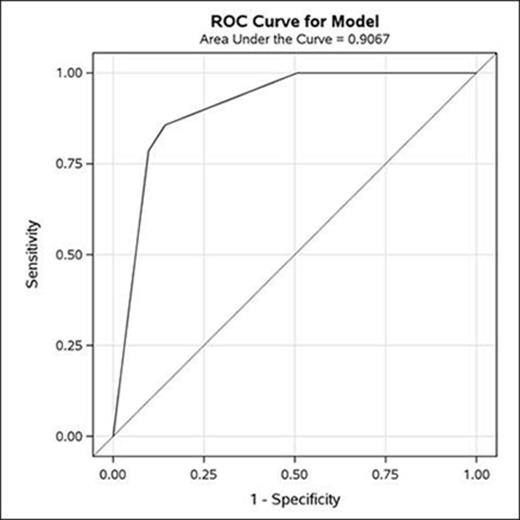
Among all patients in the study, the following variables were associated with higher odds of having HLH compared to hyperferritinemia due to an alternate cause: elevated ferritin (odds ratio [OR] 35.97, p<0.01); thrombocytopenia (OR 15.22, p<0.01), cytopenias defined by HLH-04 criteria (OR 8.04, p<0.01); elevated admission serum bilirubin (OR 1.05, p=0.02); and peak serum bilirubin (OR 1.05, p<0.01). After stepwise selection in multivariate analysis, serum ferritin ≥2,600 mcg/L and platelets ≤100 x 109/L were independently associated with HLH diagnosis (OR 24.9 and 7.8 respectively; p<0.01 for both). The area under the curve (AUC or c-statistic) ranges from 0.5 for no ability to discriminate, to 1.0 for perfect discrimination; this model has an AUC of 0.91, which shows very good discrimination between cases and controls. An adult hospitalized patient with a combination of serum ferritin ≥2,600 mcg/L and platelets ≤100x109/L predicts a ~200 fold increased likelihood of being diagnosed with HLH as compared to hospitalized adult meeting neither of these criteria (Figures 1 and 2).
CONCLUSION: In contrast to hospitalized pediatric patients, the etiology of hyperferritinemia in adults is more likely to be malignancy, bacterial infection, liver disease, and cardiac disease. We conclude that a combination of serum ferritin ≥2,600 mcg/L and platelet count ≤100 x 109/L can be used as screening criteria to help identify adult patients most likely to have HLH. The traditional 5/8 criteria to diagnose HLH in pediatric patients do not appear necessary for establishing a diagnosis of HLH in adult patients. These observations need replication in an independent data set prior to broader applicability.
Patients with ferritin > 500 mcg/L with underlying cause for elevation.
| . | Number Diagnosed (%) . |
|---|---|
| Cardiac Disease | 95 (7.2%) |
| Liver Disease | 131 (9.9%) |
| Renal Disease | 87 (6.6%) |
| Infectious | 343 (25.8%) |
| Malignancy | 373 (28.1%) |
| Autoimmune | 118 (8.9%) |
| Solid Organ Transplant | 34 (2.6%) |
| Stem Cell Transplant | 16 (1.2%) |
| Bone Marrow Failure | 9 (0.7%) |
| Shock | 39 (2.9%) |
| Idiopathic | 53 (4.0%) |
| Hemoglobinopathies | 3 (0.2%) |
| . | Number Diagnosed (%) . |
|---|---|
| Cardiac Disease | 95 (7.2%) |
| Liver Disease | 131 (9.9%) |
| Renal Disease | 87 (6.6%) |
| Infectious | 343 (25.8%) |
| Malignancy | 373 (28.1%) |
| Autoimmune | 118 (8.9%) |
| Solid Organ Transplant | 34 (2.6%) |
| Stem Cell Transplant | 16 (1.2%) |
| Bone Marrow Failure | 9 (0.7%) |
| Shock | 39 (2.9%) |
| Idiopathic | 53 (4.0%) |
| Hemoglobinopathies | 3 (0.2%) |
ROC curve for the multivariable model with ferritin > 2,600 (yes/no) and platelets below 100,000 (yes/no) in the model with a very good discrimination c-statistic of 0.91.
ROC curve for the multivariable model with ferritin > 2,600 (yes/no) and platelets below 100,000 (yes/no) in the model with a very good discrimination c-statistic of 0.91.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract