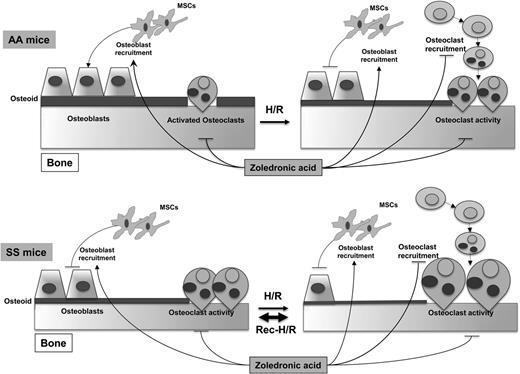
In this issue of Blood, Dalle Carbonare et al report that osteoclast activation in combination with suppressed osteoblast function is central to sickle bone disease in a murine model and that zoledronic acid prevents hypoxia/reperfusion-related exacerbation of these processes.1
Proposed pathways of sickle cell bone disease in mice. Hemoglobin SS mice display increased osteoclast activity and reduced osteoblastogenesis, resulting in reduced osteoid formation compared with hemoglobin AA mice. Hypoxia/reoxygenation stress depresses osteoblastogenesis and increases osteoclast activity, promoting bone impairment. Recurrent stress further worsens the imbalance between osteoblastogenesis and osteoclastogenesis, resulting in bone loss and severe bone impairment. Zoledronic acid blocks osteoclast activity and osteoclastogenesis and is associated with increased osteoblast recruitment and osteoblastogenesis, preventing sickle bone disease. H/R, hypoxia reoxygenation stress; MSCs, mesenchymal stem cells. See Figure 6 in the article by Dalle Carbonare et al on page 2320.
Proposed pathways of sickle cell bone disease in mice. Hemoglobin SS mice display increased osteoclast activity and reduced osteoblastogenesis, resulting in reduced osteoid formation compared with hemoglobin AA mice. Hypoxia/reoxygenation stress depresses osteoblastogenesis and increases osteoclast activity, promoting bone impairment. Recurrent stress further worsens the imbalance between osteoblastogenesis and osteoclastogenesis, resulting in bone loss and severe bone impairment. Zoledronic acid blocks osteoclast activity and osteoclastogenesis and is associated with increased osteoblast recruitment and osteoblastogenesis, preventing sickle bone disease. H/R, hypoxia reoxygenation stress; MSCs, mesenchymal stem cells. See Figure 6 in the article by Dalle Carbonare et al on page 2320.
Osteoporosis and osteopenia are common findings in sickle cell disease (SCD) patients.2 In fact, altered bone metabolism begins in early childhood,3 and >65% of adult patients have low bone mass density.4 Previous investigations reported that markers of bone turnover are increased in SCD patients5 and that serum levels of tartrate-resistant acid phosphatase (TRACP) type 5b, an enzyme produced specifically by activated bone-resorbing osteoclasts,6 are markedly elevated in SCD adults.7 Interleukin (IL)-6 concentrations were associated with higher TRACP 5b concentrations in the latter study, pointing to the possibility of IL-6 induction of receptor activator for nuclear factor-ĸB ligand (RANKL) as a potential mechanism for osteoclast activation and, subsequently, bone resorption.8
The work by Carbonare et al in a humanized mouse model of SCD provides strong mechanistic explanations for the previous associative findings and contributes substantially to our understanding of the genesis of sickle bone disease. These investigators found that under normoxia, osteoclast activity was increased and osteoblast activity was reduced, and that with repeated hypoxia/reperfusion episodes these abnormalities were magnified.
The elegant experiments included histomorphometry to assess bone homeostasis and turnover, measurement of the ratio of osteoclasts to tissue area and assay of osteoblastic colony forming units, and determination of osteoclastic and osteoblastic markers in bone tissue using reverse transcriptase-polymerase chain reaction. The investigators first reported experiments done under normoxic conditions. Bone loss was present as was increased expression of the osteoclastic marker RankL.8 In turn, bone genesis was impaired as indicated by a reduction in the number of osteoblastic colony forming units and downregulation of the osteogenic markers Runx2 and Sparc. Runx2 is a transcription factor that plays a role in activating Sparc, which encodes osteonectin, a glycoprotein secreted by osteoblasts during bone formation.
The investigators then repeated experiments after single or repeated episodes of hypoxia followed by reoxygenation to mimic acute vaso-occlusive crises. Repeated hypoxia/reperfusion stress further worsened bone structure and increased bone turnover. In addition to increased expression of the osteoclast activity markers RankL and Cathepsin k, repeated hypoxia/reperfusion stress was associated with osteoclast recruitment as indicated by increased expression of Rank and with upregulation of Il6 as a proresorptive cytokine. Repeated stress was also associated with further suppression of the osteogenic lineage markers Runx2 and Sparc.
Zoledronic acid is an amino-bisphosphonate that inhibits osteoclast activity and osteoclastogenesis and promotes osteogenic differentiation.9 The investigators tested the ability of this agent to interfere with the development of sickle bone disease in the mouse model. Zoledronic acid markedly inhibited osteoclast activity and recruitment and promoted the osteogenic lineage. Given before the induction of recurrent hypoxia/reperfusion stress, it was effective in preventing bone impairment.
The authors summarize their findings in a schematic diagram (see figure). These results are significant in that they point to a mechanism for the development of sickle bone disease in the mouse model that fits well with previous studies in humans using only indirect markers of the pathologic process underway in the bones. The results should point the way to further translational studies for the understanding, prevention, and treatment of sickle bone disease. How the findings relate to the development of avascular necrosis of joints that is such a painful and disabling problem for a subset of patients should also be investigated. The potential for zoledronic acid and other bisphosphonates to prevent sickle bone disease in the mouse model is exciting given the established utility of these agents for treating osteoporosis in human populations. A note of caution in this regard is that it will be important to consider the potential for renal toxicity with bisphosphonates given the high prevalence of chronic kidney disease in adult SCD patients.10
Conflict-of-interest disclosure: V.R.G. has served as a consultant for Incyte Corporation and La Jolla Pharmaceuticals in the last year.