In this issue of Blood, Omarova et al show that fibrinogen, particularly the γ′ variant, increases the anticoagulant effect of activated protein C in plasma.1
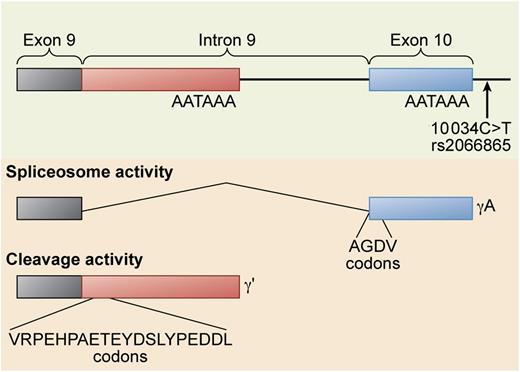
Alternative processing of the FGG gene pre-mRNA. The FGG 10 034C>T SNP (rs2066865) results in lower levels of γ′ fibrinogen. This SNP may affect the relative rate of spliceosome activity that cleaves out the ninth intron to form the γA isoform vs polyadenylation and cleavage activity that polyadenylates within the intron and cleaves the mRNA at the 3′ end of the polyadenylation site to form the γ′ isoform. Professional illustration by Xavier Studio.
Alternative processing of the FGG gene pre-mRNA. The FGG 10 034C>T SNP (rs2066865) results in lower levels of γ′ fibrinogen. This SNP may affect the relative rate of spliceosome activity that cleaves out the ninth intron to form the γA isoform vs polyadenylation and cleavage activity that polyadenylates within the intron and cleaves the mRNA at the 3′ end of the polyadenylation site to form the γ′ isoform. Professional illustration by Xavier Studio.
Whether γ′ (pronounced gamma prime) fibrinogen is prothrombotic or antithrombotic is a somewhat contentious issue. The concentration of γ′ fibrinogen and the ratio of γ′ fibrinogen to total fibrinogen is associated with both arterial and venous thrombosis, but not always in the same direction.2,3 Fibrinogen is a 6-chain protein containing 2 copies each of the Aα, Bβ, and γ chains. γ′ fibrinogen arises from an alternative messenger RNA (mRNA) processing event4 in the γ chain pre-mRNA (see figure). Approximately 7% of total fibrinogen contains a γ′ chain, although the concentration range is quite broad, unlike most coagulation factors, with a reference interval of 8.8 to 55.1 mg/dL.5 Although epidemiology studies of arterial thrombosis demonstrate consistently that elevated levels of γ′ fibrinogen are associated with cardiovascular disease, the same cannot be said of epidemiology studies of venous thrombosis and thrombotic microangiopathy. Some studies show an association with γ′ fibrinogen levels, whereas other studies show an inverse association. This is the origin of the debate as to whether γ′ fibrinogen is prothrombotic or antithrombotic.
There is convincing biochemical evidence on both sides of the argument. On the one hand, human γ′ fibrinogen is not prothrombotic in mouse models.6 In addition, γ′ fibrinogen has “antithrombin I” activity,7 an inhibitory activity toward thrombin that decreases its ability to activate coagulation factors and platelets in vitro. This activity is due to a high-affinity binding site on the γ′ chain for thrombin exosite II.3 Furthermore, the FGG H2 haplotype results in decreased γ′ fibrinogen levels and is associated with venous thrombosis.2 The functional single nucleotide polymorphism (SNP) in the FGG H2 haplotype that decreases γ′ fibrinogen levels is FGG 10 034C>T, rs2066865 (see figure). This tends to support the hypothesis that low γ′ fibrinogen levels are prothrombotic, with the caveat that FGG 10 034C>T may be a proxy for another functional SNP located elsewhere within the FGG H2 haplotype. In particular, FGA Thr312Ala (rs6050) is also in the FGG H2 haplotype, and this SNP results in fibrinogen molecules that form stiffer clots with increased α chain cross-linking.8
On the other side of the argument, γ′ fibrinogen forms fibrin clots with an altered clot architecture containing thinner, more numerous fibers that are less permeable, mechanically stronger, and highly resistant to fibrinolysis.3 γ′ fibrinogen levels are also significantly associated with inflammation,3 a well-known risk factor for cardiovascular disease. In addition, active thrombin binds to the γ′ chain and is protected from inactivation by antithrombin III,9 providing a potential reservoir of reversibly clot-bound thrombin.
The study by Omarova et al1 provides more support for the theory that γ′ fibrinogen is antithrombotic. Their findings are complicated by the fact that the presence of even unfractionated fibrinogen in plasma results in greater thrombin generation than in fibrinogenemic plasma, presumably from a low-affinity thrombin-binding site in fibrinogen that protects thrombin from antithrombin III inactivation. γ′ fibrinogen supports more thrombin generation under these conditions because its high-affinity thrombin-binding site confers even greater protection from antithrombin III.9 However, in the presence of activated protein C, this effect is reversed; adding γ′ fibrinogen to plasma results in less thrombin generation. This effect is not due to increased protein C activation in the presence of the γ′ chain because the authors demonstrate that the γ′ chain actually inhibits protein C activation by thrombin/thrombomodulin. They postulate that the mechanism underlying the decreased thrombin generation is the inhibition of factor V10 and factor VIII3 activation, 2 thrombin activities that are known to be blocked by γ′ fibrinogen. Furthermore, the authors suggest that the unique C-terminal peptide of γ′ fibrinogen, VRPEHPAETE(sY)DSL(sY)PEDDL (see figure), could be used as a pharmacological intervention to counteract activated protein C resistance, providing the promise of a novel therapy for venous thrombosis.
Conflict-of-interest disclosure: Oregon Health & Science University (OHSU) and David Farrell have a significant interest in Gamma Therapeutics, a company that may have a commercial interest in the results of this research and technology. This potential individual and institutional conflict of interest has been reviewed and managed by OHSU.