In this issue of Blood, Gallipoli et al show that combined targeting of BCR/ABL1 and Janus kinase 2 (JAK2) by 2 established kinase inhibitors, nilotinib (NI) and the JAK1/2 kinase inhibitor ruxolitinib (RUX), results in synergistic growth inhibition in immature CD34+ stem and progenitor cells obtained from patients with chronic myeloid leukemia (CML).1
The JAK2–signal transducer and activator of transcription 5 (STAT5) axis is an emerging target of therapy in stem cell–derived, myeloid neoplasms. During the past few years, several targeting concepts around JAK2 have been proposed in myeloid neoplasms, mostly in JAK2 V617F–transformed malignancies. As a result, several different, more or less specific, JAK2 inhibitors have been developed. CML is a stem cell neoplasm defined by the BCR/ABL1 oncoprotein that acts as a dominant driver in the chronic phase (CP) of the disease. A number of previous and more recent data suggest that the JAK2-STAT5 pathway plays an important role in the biology and evolution of CML.2,3 More recently, JAK2 and STAT5 have been described as potential therapeutic targets in leukemic stem cells (LSCs) in CML1,4 which is important as CML LSCs exhibit intrinsic resistance against BCR/ABL1-targeting tyrosine kinase inhibitors (TKIs) such as imatinib. In addition, acquired resistance may occur in more malignant LSC subclones which can in turn lead to an overt relapse in these patients.
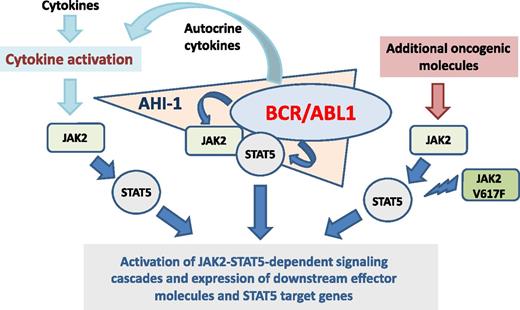
BCR/ABL1 initiates a number of prooncogenic downstream pathways that act together in a complex signaling network and thereby promotes growth and survival of neoplastic cells and thus disease evolution. Among several other pathways, BCR/ABL1 also activates JAK2 and STAT5.3,5,6 Whereas the C terminus of BCR-ABL1 binds JAK2 physically, the Src homology 2 domain of BCR-ABL1 is involved in the phosphorylation of JAK2.2,3 Once activated, JAK2 itself initiates a number of downstream molecules, including STAT3 and STAT5. In addition, JAK2 regulates MYC expression.5 Furthermore, activated JAK2 can block tyrosine protein phosphatase 2A activity.6 BCR/ABL1 also activates STAT5 directly and thus independent of JAK2.7 However, despite its ability to initiate multiple signaling cascades, BCR/ABL1 alone may not be a fully transforming molecule, but requires additional cooperating prooncogenic triggers to cause CML. Moreover, regarding survival and proliferation, CML LSCs may not be dependent on BCR/ABL1 in the same way as more mature cells in the leukemic clone. Based on these observations, research is focusing on BCR/ABL1-independent pathways and molecules.
The JAK2-STAT5 axis is considered a disease-promoting pathway that acts downstream of BCR/ABL1 but also independent of BCR/ABL1 in CML cells.5-8 Especially in CML LSCs and in TKI-resistant cells, JAK2 and STAT5 may be expressed and activated independent of BCR/ABL1 and may play an important role in growth and survival of LSCs and thus disease evolution (see figure).8,9 Moreover, JAK2 may be involved in growth factor-dependent signaling in LSCs (see figure). It has also been described that high STAT5 levels in CML cells correlate with resistance against imatinib.8 All in all, the critical roles of JAK2 and STAT5 become most evident when the disease progresses in TKI-resistant subclones.
Little is known so far about additional drivers and prooncogenic pathways that contribute to BCR/ABL-independent expression and activation of JAK2 and/or STAT5 in CML cells. In a smaller group of patients, the JAK2 mutation V617F has been identified. Clinical observations and in vitro data suggest that both mutants are usually expressed in different LSC fractions. However, there may be other additional pathways and drivers that promote the expression and/or activation of JAK2 and/or STAT5 in CML LSCs. Deep-sequencing strategies are expected to reveal these additional drivers and help understand how the JAK2-STAT5 pathway contributes to disease progression and drug resistance in advanced CML.
As mentioned, recent data suggest that the JAK2-STAT5 pathway plays a particular role in survival and proliferation of CML LSCs.1,9 However, little is known about the underlying mechanisms and molecular interactions. One important point may be that CML LSCs express considerable amounts of Abelson helper integration site-1 (AHI-1), a prooncogenic adaptor that stabilizes BCR-ABL1 by recruiting JAK2 (see figure).4,9 The resulting signaling complex may be critically involved in LSC survival and growth but also in resistance against TKI.4,9 The consecutive activation of downstream STAT5 may play an essential role in oncogenesis through multiple mechanisms and STAT5 target genes. One additional important aspect is that STAT5 triggers the formation of reactive oxygen species which in turn leads to DNA damage and the acquisition of additional lesions in CML LSCs.10 These observations also suggest that although BCR/ABL1 alone is not a fully transforming oncoprotein, long-term effects caused by this driver lesion through activated JAK2 and STAT5 in neoplastic cells may well result in a full-blown malignancy.
Although the important roles of JAK2 and STAT5 in LSC growth and survival and thus disease evolution are well appreciated, it remains unclear whether targeting of either JAK2 or STAT5 is meaningful and can be achieved using currently available drugs. In this regard, it is noteworthy that BCR/ABL1 transforms myeloid stem cells in mice independent of JAK2.7 With regard to JAK2, it also remains unknown whether the available blockers, such as RUX, exhibit sufficient specificity and potency. However, these TKI may also inhibit the activation of other key kinases, including JAK1 and BCR/ABL1.7 The data of Gallipoli and colleagues show that NI and RUX synergize with each other in inhibiting the proliferation of CD34+ cells in patients with CML.1 So far, it remains unclear whether this synergistic effect was obtained by specifically targeting BCR/ABL1 and JAK2 in CML LSCs. Alternatively, the drug combination suppressed also other kinase or nonkinase targets or even BCR/ABL1 in these cells. Whatever the basis of the drug-combination effect is, the data presented are encouraging and suggest that this combination should now be tested in clinical trials in patients with advanced CML. With regard to STAT5, the major problem is that no specific and potent inhibitors have been developed far enough to reach clinical application. In addition, STAT5 inhibition in LSCs alone may not be sufficient as other STAT molecules, such as STAT3, may compensate a loss or suppression of STAT5. Because STAT3 is also downstream of JAK2 and has also been implicated in the pathogenesis of CML, targeting of JAK2 may be a more logical approach as it suppresses the activity of both STAT3 and STAT5 in CML (stem) cells.
In summary, JAK2 may serve as a novel potential target of therapy in Ph+ CML. Because the JAK2-STAT pathway appears to be relevant to LSC survival and evolution, targeting of the pathway by JAK2 blockers may increase responses to BCR/ABL1 TKI and hopefully cure rates in patients with advanced CML. The data presented by Gallipoli et al support this novel concept.1 Clinical trials using such drug combinations are now warranted to test this concept in patients with CML.
We thank Veronika Sexl and Richard Moriggl for critical reading of the text of the commentary and for their helpful discussions.
Conflict-of-interest disclosure: The author declares no competing financial interests.