Abstract
Although most circulating iron in blood plasma is destined for erythropoiesis, the mechanisms by which erythropoietic demand modulates the iron supply (“erythroid regulators”) remain largely unknown. Iron absorption, plasma iron concentrations, and tissue iron distribution are tightly controlled by the liver-produced hormone hepcidin. During the last decade, much progress has been made in elucidating hepcidin regulation by iron and inflammation. This review discusses the less understood mechanisms and mediators of hepcidin suppression in physiologically and pathologically stimulated erythropoiesis.
Introduction
Iron is an essential functional component of erythrocyte hemoglobin, therefore the production of erythrocytes requires the timely delivery of iron to erythroid precursors. The process of erythropoiesis is by far the largest consumer of iron in the body, with red cells containing two-thirds of the total body iron. Five liters of human adult blood contains ∼2.5 × 1013 erythrocytes that have an average lifespan of 120 days. Steady-state erythropoiesis involves the production of 200 billion new red blood cells per day or 2.4 million per second.1 This requires a daily supply of 20 to 25 mg of iron, in the form of holotransferrin. The transferrin compartment contains only about 3 mg of iron at any time, and turns over every few hours. Most of the iron for erythropoiesis in humans is supplied by the recycling of senescent red blood cells by macrophages, a process known as erythrophagocytosis which provides ∼20 to 25 mg of iron per day. In the steady state, intestinal absorption of dietary iron amounts to only 1 to 2 mg per day (<0.05% of total body iron), sufficient to compensate for the small amount of iron lost from the body through desquamation of epithelial cells in the intestine and the skin or minor blood loss.
Iron supply to the marrow comes under particular strain after hemorrhage, hemolysis, and other conditions that trigger stress erythropoiesis. When erythrocytes are lost, critical signals facilitating the provision of iron for restorative erythropoiesis are expected to occur within hours, as rapid recovery of red cell mass and oxygen carrying capacity confers obvious evolutionary advantages. During maximally stimulated erythropoiesis, hemoglobin synthesis and therefore iron consumption by the bone marrow can increase up to 10-fold2 and must be matched by a comparable increase in dietary iron absorption and in the release of iron from stores. Failure to match iron supply to demand would rapidly deplete the small iron-transferrin compartment and limit the iron supply not only for hemoglobin synthesis but also for all other iron-dependent processes. However, the mechanism by which erythropoiesis modulates iron homeostasis, historically referred to as the “erythroid regulator,”2 is poorly understood.
Hepcidin: the link between erythropoiesis and iron regulation
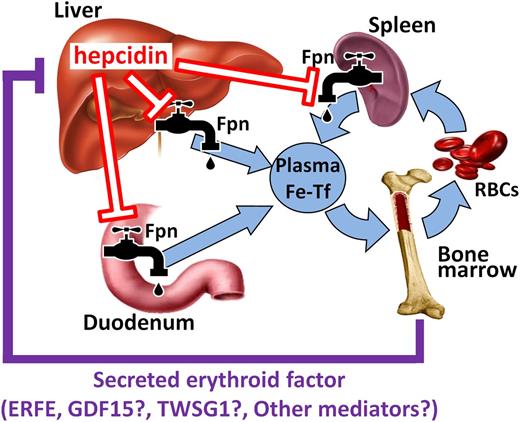
The liver-produced hormone hepcidin has emerged as the main circulating regulator of iron absorption and tissue distribution,3 and represents the distal component of the “erythroid regulator” mechanism. Hepcidin acts by binding to ferroportin, the sole known cellular iron exporter, leading to ubiquitination, internalization, and degradation of ferroportin in lysosomes.4 Under the influence of high hepcidin concentrations, ferroportin is depleted from cell membranes, iron is retained in iron-exporting cells, and the flow of iron into plasma is decreased. Conversely, when hepcidin production is reduced, stabilization of ferroportin at the cellular membrane promotes the absorption of dietary iron in the duodenum, increases the release of iron from macrophages that recycle old erythrocytes and other cells, and allows the mobilization of stored iron from hepatocytes (Figure 1). Reflecting the greatly increased demand for iron, hepcidin is suppressed in conditions associated with accelerated erythropoiesis, including anemias caused by bleeding, hemolysis, or iron deficiency, and in hereditary anemias with ineffective erythropoiesis.3 The mechanism(s) involved in these responses are only beginning to be understood.
Hepcidin coordinates iron supply for erythropoiesis. Hepcidin controls major iron flows into plasma by causing degradation of its receptor ferroportin. After erythropoietic stimulation, erythroid precursors secrete 1 or more mediators (erythroid factors) that suppress hepcidin production in the liver, resulting in increased iron supply to the bone marrow. ERFE has been proposed to mediate hepcidin suppression in both physiological (eg, after hemorrhage) and pathological conditions (eg, ineffective erythropoiesis), whereas GDF15 and TWSG1 may only play a role in iron-loading anemias. Other erythroid regulators may exist but have yet to be identified.
Hepcidin coordinates iron supply for erythropoiesis. Hepcidin controls major iron flows into plasma by causing degradation of its receptor ferroportin. After erythropoietic stimulation, erythroid precursors secrete 1 or more mediators (erythroid factors) that suppress hepcidin production in the liver, resulting in increased iron supply to the bone marrow. ERFE has been proposed to mediate hepcidin suppression in both physiological (eg, after hemorrhage) and pathological conditions (eg, ineffective erythropoiesis), whereas GDF15 and TWSG1 may only play a role in iron-loading anemias. Other erythroid regulators may exist but have yet to be identified.
Disordered interaction between iron metabolism and erythropoiesis
Disruption of homeostatic mechanisms regulating hepcidin can lead to impaired erythropoiesis. Increased circulating hepcidin causes or contributes to iron-restrictive anemia in inflammatory disorders, chronic kidney diseases, cancer, and iron-refractory iron deficiency anemia (IRIDA).3 Multiple pathogenic mechanisms can increase hepcidin concentrations in blood plasma. In inflammatory disorders, hepcidin synthesis is stimulated by proinflammatory cytokines, most prominently interleukin-6. In chronic kidney disease, not only inflammation but also decreased renal clearance of hepcidin peptide seem to contribute to higher hepcidin levels. In the genetic disease IRIDA, hepcidin is increased due to loss-of-function mutations in matriptase-2 (aka TMPRSS6), a negative regulator of hepcidin transcription. In all these conditions, the increase in plasma hepcidin concentrations is the cause of hypoferremia and inadequate iron supply for erythropoiesis.
Conversely, pathological erythropoiesis can cause iron disorders. Ineffective erythropoiesis suppresses hepcidin production,5,6 which causes hyperabsorption of dietary iron and iron overload even in the absence of erythrocyte transfusions.5 Genetic disorders with ineffective erythropoiesis include thalassemias and congenital dyserythropoietic anemias. In these conditions, erythrocyte precursors massively expand but do not mature into erythrocytes and mostly undergo apoptosis at the erythroblast stage.7 Transfusions partially relieve the erythropoietin (EPO)-driven expansion of ineffective erythropoiesis and raise hepcidin concentrations,6 but still cause iron overload owing to the iron content of transfused blood. In thalassemia, iron overload may further aggravate ineffective erythropoiesis by stimulating erythroblast reactive oxygen species production and increasing the imbalance between globin chains.8 Whether the same or overlapping hepcidin suppressor(s) function both in ineffective erythropoiesis and in physiological recovery from hemorrhage is unknown but considered likely as the 2 situations share the common feature of high levels of EPO and expansion of erythroblast populations.
Hepcidin regulation by erythropoiesis
Increased EPO is the primary stimulus for erythropoietic activity, and both EPO concentrations and indicators of erythropoietic activity are inversely correlated with hepcidin expression in patients with β-thalassemia.5,6 However, EPO does not seem to have a direct effect on hepatic hepcidin production: EPO injections in mice with ablated bone marrow failed to suppress hepcidin.9,10 The transcription factor hypoxia-inducible factor (HIF), a key mediator of cellular adaptation to hypoxia, directly regulates renal and hepatic EPO synthesis during anemia, and was also considered a candidate regulator of hepcidin transcription. Nevertheless, recent evidence indicates that hepcidin suppression by the HIF pathway is indirect, through the EPO-mediated stimulation of erythropoiesis.11,12 Increased iron utilization after EPO administration is also not the mediator of hepcidin suppression because the dramatic hepcidin decrease precedes any changes in transferrin saturation or other known iron-related parameters.13 Rather, accumulating evidence suggests that the erythroid regulator of hepcidin is a circulating factor produced by erythroid precursors in the bone marrow in response to EPO (Figures 1-2).
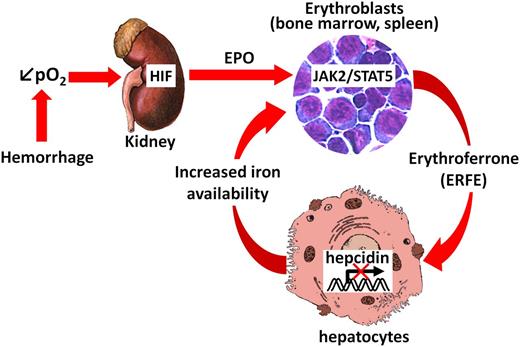
Proposed mechanism of action of the erythroid regulator ERFE. After blood loss, decreased oxygen delivery is sensed in the kidneys, stabilizing HIF and inducing EPO production. Binding of EPO to its receptors on erythroid progenitors in the bone marrow and the spleen leads to the rapid production of ERFE via the JAK2/STAT5 signaling pathway. ERFE is then secreted into the circulation and acts directly on hepatocytes to signal for hepcidin suppression in order to increase the iron flow into plasma for new red blood cell synthesis.
Proposed mechanism of action of the erythroid regulator ERFE. After blood loss, decreased oxygen delivery is sensed in the kidneys, stabilizing HIF and inducing EPO production. Binding of EPO to its receptors on erythroid progenitors in the bone marrow and the spleen leads to the rapid production of ERFE via the JAK2/STAT5 signaling pathway. ERFE is then secreted into the circulation and acts directly on hepatocytes to signal for hepcidin suppression in order to increase the iron flow into plasma for new red blood cell synthesis.
Candidate erythroid factors regulating hepcidin
Two members of the transforming growth factor-β superfamily, growth differentiation factor 15 (GDF15) and twisted gastrulation (TWSG1), have been proposed as pathological suppressors of hepcidin in ineffective erythropoiesis.14,15 Although synthesized in many other tissues, these factors are also expressed during late (GDF15) and early (TWSG1) stages of human erythroblast differentiation ex vivo.14 GDF15 is known to play roles in inflammation, cancer, cardiovascular diseases, and obesity through incompletely understood mechanisms,16 whereas TWSG1 modulates bone morphogenetic protein activity during embryonic development and in multiple adult tissues.17
In vitro, high concentrations of GDF15 or TWSG1 suppressed hepcidin expression in primary hepatocytes or hepatocyte cell lines.14,15 However, the role of GDF15 and TWSG1 in hepcidin suppression in vivo is less clear. Twsg1 messenger RNA (mRNA) expression is increased in the spleen, liver, and, to a much lesser extent, the bone marrow of the murine β-thalassemia models,12,15,18 but the contribution of increased TWSG1 to pathological hepcidin suppression is unknown. It is also not known whether TWSG1 is increased in human patients with ineffective erythropoiesis. TWSG1 does not appear to be involved in physiological hepcidin suppression by increased erythropoiesis in mice: Twsg1 mRNA was unchanged in the bone marrow and spleen of Vhl−/− mice that have high Epo levels,12 or in wild-type (WT) mice after Epo administration,12 or in WT mice after severe blood loss.19
GDF15 was proposed to suppress hepcidin in β-thalassemia or congenital dyserythropoietic anemia in humans as its blood levels are high in ineffective erythropoiesis.14,20 However, direct evidence for the role of GDF15 is still lacking. One of the difficulties is the species-specific difference in the tissue expression and regulation of Gdf15. Unlike their human counterparts, mouse erythoblasts do not produce Gdf15 during differentiation in vitro.21 Furthermore, Gdf15 is not increased in murine β-thalassemia,12,18,19 in contrast to human disease. This suggests that mouse models may not be appropriate to study the role of Gdf15 in hepcidin regulation. However, some parallels have been reported between human and mouse Gdf15 regulation. Elevated EPO was associated with higher Gdf15 expression in certain mouse models: Vhl−/− mice with high EPO levels or WT mice injected with EPO both increased Gdf15 expression in bone marrow and spleen, although this translated into only a small increase in serum concentrations.12 Similarly, small increases in serum GDF15 were observed following ascent to high altitude22 but these levels were much lower than the concentrations observed in thalassemia patients. Single injection of EPO in human volunteers resulted in either no induction of GDF1513 or a small induction when a large EPO dose was used.23 In both studies, hepcidin levels were dramatically decreased. Thus, GDF15 does not appear to be necessary for acute hepcidin regulation by EPO.
Only one study directly assessed the role of Gdf15 in hepcidin regulation by using Gdf15 knockout mice.19 Hepcidin response to phlebotomy was assessed on day 3, after removing a total of 0.8 mL of blood over the first 2 days. WT mice increased Gdf15 mRNA in the bone marrow approximately threefold, but hepcidin was similarly suppressed between Gdf15-deficient and WT mice. This suggests that GDF15 is not necessary for physiological suppression of hepcidin after hemorrhage in mice, although a more detailed time-course analysis would be necessary to eliminate the role of Gdf15 in acute hepcidin suppression (within hours after hemorrhage). In humans, subjects with iron deficiency24 or iron-deficiency anemia25,26 had normal or only marginally elevated GDF15,27 further suggesting that GDF15 is not a physiological regulator of hepcidin. Similarly, GDF15 did not appear to be a regulator of hepcidin in myelodysplastic syndrome28 or chronic myeloproliferative disease.29 Thus, direct involvement of GDF15 or TWSG1 in hepcidin regulation in humans still remains to be demonstrated.
Erythroferrone, a new regulator of hepcidin
We recently described the identification of the hormone erythroferrone (ERFE), a new erythroid regulator of hepcidin that belongs to the C1q–tumor necrosis factor–related family of proteins.30 In response to EPO, ERFE is rapidly produced by erythroid precursors in the bone marrow and the spleen via signal transducer and activator of transcription 5 (Stat5)30 (Figure 2). The canonical Janus kinase 2 (Jak2)/Stat5 signaling pathway is a known mediator of EPO response and stress erythropoiesis.31 Phlebotomy or EPO administration in ERFE-knockout mice failed to suppress hepcidin, demonstrating that ERFE is absolutely necessary for acute hepcidin response to increased erythroid activity.30 However, our study design focused only on early responses (first 24 hours) and we therefore cannot exclude that other erythroid regulators may exist and intervene later on. Lentiviral overexpression of ERFE in mice confirmed its suppressive effect on hepcidin. Treatment of primary mouse hepatocytes with recombinant ERFE indicated that ERFE acts directly on the liver to decrease hepcidin transcription, although the pathways involved, including the ERFE receptor, are still unknown. Interestingly, ERFE’s role is restricted to stress erythropoiesis and does not seem to affect steady-state erythropoiesis as adult Erfe-deficient mice had normal hematologic and iron parameters. However, even the mild stress of expanding erythropoiesis in rapidly growing juvenile mice was sufficient to cause transient anemia in ERFE-deficient mice.
ERFE mRNA levels were also remarkably elevated in the bone marrow and the spleen of a mouse model of β-thalassemia intermedia (Hbbth3/th3)30 suggesting that Erfe could be a pathological suppressor of hepcidin in ineffective erythropoiesis. Importantly, ablation of ERFE in thalassemic mice restored hepcidin levels to normal, and significantly reduced the liver iron content and serum iron concentration compared with the thalassemic mice. The function of ERFE in humans remains to be investigated, although ex vivo studies confirm that EPO induces ERFE in human erythroid precursors.30 Further work is necessary to confirm whether ERFE is the long-sought erythroid regulator responsible for hepcidin suppression and iron overload in patients with hereditary iron-loading anemias.
Novel approaches to treat disorders of erythropoiesis
Considering the role of increased hepcidin in iron-restricted anemias, it is not surprising that hepcidin has become a promising target for novel therapeutic approaches.32 Multiple agents directed at lowering hepcidin production or interfering with hepcidin peptide activity are under development, and several clinical trials are already under way. Potentiating or mimicking the suppressive effect of natural erythroid regulators of hepcidin could provide additional therapeutic options for overcoming iron restriction in inflammatory disorders, chronic kidney disease, or cancer.
At the other end of the spectrum, causing mild iron restriction by increasing hepcidin in iron-loading anemias may produce surprising therapeutic benefits. In mouse models of β-thalassemia, moderate increase in hepcidin, caused either by transgenic hepcidin overexpression, knockdown of the negative hepcidin regulator matriptase 2, or administration of hepcidin agonists, resulted not only in less iron loading but also improvement in anemia.8,32 The hematologic benefits of mild iron restriction seem to be the result of decreased α-globin precipitation, lower reactive oxygen species levels, and decreased apoptosis.8 Similar results may be expected from interfering with overactive erythroid regulators that suppress hepcidin in ineffective erythropoiesis (eg, ERFE).
The interface between erythropoiesis and iron metabolism continues to yield exciting and therapeutically promising discoveries. Just recently, a therapy based on activin receptor ligand traps was shown to improve anemia and diminish iron overload in mouse models of β-thalassemia and myelodysplastic syndrome by inhibiting GDF11, a novel regulator of erythropoiesis.33,34 It is not yet known whether Gdf11 directly regulates iron homeostasis.
Important scientific advances made in recent years have brought increased understanding of the intimate relationship between erythropoiesis and iron homeostasis that will eventually benefit patients suffering from iron disorders and anemias.
Acknowledgments
This work was supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant R01 DK90554 (E.N.) and the American Society of Hematology (ASH) scholar award (L.K.).
Authorship
Contribution: L.K. and E.N. wrote the paper.
Conflict-of-interest disclosure: E.N. is a cofounder, shareholder, and officer of Intrinsic LifeSciences, a company developing hepcidin diagnostics, and shareholder in Merganser Biotech, engaged in the development of hepcidin-based therapeutics. The remaining author declares no competing financial interests.
Correspondence: Elizabeta Nemeth, Department of Medicine, UCLA, 10833 LeConte Ave, CHS 37-131, Los Angeles, CA 90095; e-mail: enemeth@mednet.ucla.edu.