Abstract

BACKGROUND: Ruxolitinib (RUX) is a potent JAK1/JAK2 inhibitor that has demonstrated rapid and durable reductions in splenomegaly, improvements in MF-related symptoms and quality-of-life measures, and prolonged survival in 2 phase 3 studies (COMFORT-I and -II). RUX is generally well tolerated, with low rates of nonhematologic adverse events (AEs). As expected based on the mechanism of action of JAK1/JAK2 inhibition, anemia and thrombocytopenia are frequent, but manageable, AEs with RUX. Anemia is a known adverse prognostic factor in MF patients (pts) not receiving JAK inhibitor therapy; however, the prognostic impact of anemia in pts receiving RUX has not been determined. Here, we characterize the dynamics of hemoglobin (Hb) changes with RUX treatment and assess prognostic effects.
METHODS: Data from the COMFORT studies, in which pts with MF were treated with RUX 15 or 20 mg bid based on their baseline platelet counts (PLTs), were used to define Hb changes during the course of treatment. Baseline characteristics were evaluated against observed patterns of Hb changes. A multivariate Cox proportional hazards regression model was used to assess the impact of baseline Hb on overall survival (OS) and to evaluate the effect of Hb decreases at wk 12 on observed OS to assess whether on-treatment Hb changes with RUX retain the same prognostic effects as pre-treatment Hb levels in pts with MF in the landmark analysis at wk 12. The models were fit by treatment arm and were adjusted for baseline white cell count, PLT count, age, MF subtype, sex, and spleen length.
RESULTS: 301 pts were randomized to RUX and 227 pts to the control group (placebo, n = 154; best available therapy [BAT], n = 73). Median (range) Hb at baseline was 105 g/L (66-170 g/L) and 106 g/L (65-162 g/L) in the RUX groups of COMFORT-I and -II, 105 g/L (35-173 g/L) in the placebo group, and 103 g/L (54-154 g/L) in the BAT group.
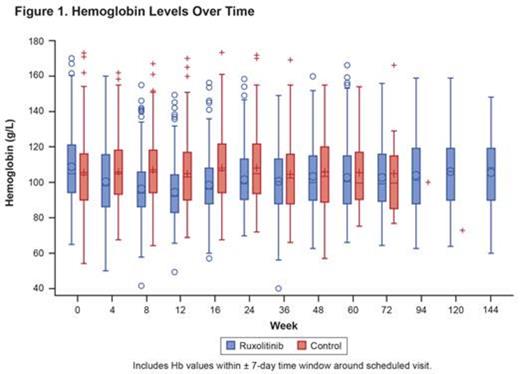
At wk 12, 82% of pts (247/301) had an Hb decrease of ≥ 3 g/dL from baseline with RUX. This ≥ 3-g/dL decrease was seen in pts with baseline Hb levels of ≥ 10 and < 10 g/dL (72% and 88%, respectively). Baseline spleen volume was also similar for pts with Hb decreases of ≥ 3 and < 3 g/dL, respectively. In pts who did not receive transfusions up to wk 12, mean spleen volume reductions from baseline at wk 24 were −30%, −32%, and −26% for pts with no decrease (n = 14), ≥ 3-g/dL decrease (n = 119), and < 3-g/dL decrease (n = 7) in Hb, respectively. Higher dose intensity during the first 12 wk of RUX treatment not only correlated with more pronounced spleen responses but was also associated with larger decreases in Hb. As reported elsewhere, following the nadir at wk 12, mean Hb levels in the overall population increased to levels similar to those in the control arm (Figure 1). Hb improvement beyond wk 12 was observed despite maintaining pre-nadir dose intensities. Analyses regarding the correlation of reticulocyte count with on-treatment Hb changes will be presented.
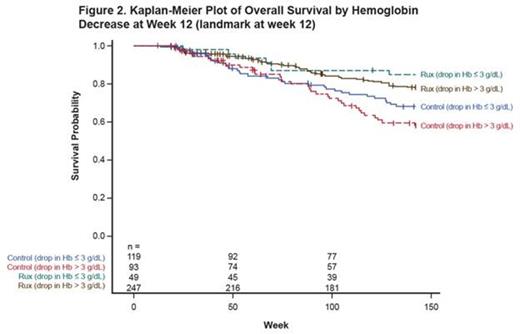
At the 3-year update, in multivariate models for nontransfused pts, baseline Hb level (per 1 g/dL) was prognostic for OS in the control arm (hazard ratio [HR] = 0.66; 95% CI, 0.54-0.81) but not in the RUX arm (HR = 0.94; 95% CI, 0.74-1.20). Additionally, Hb decreases of ≤ 3 g/dL vs > 3 g/dL at wk 12 were not associated with OS in either arm (control, HR = 0.72; 95% CI, 0.34-1.49; RUX, HR = 0.85; 95% CI, 0.27-2.69). These findings were reproducible in the overall population independent of transfusion requirements (Figure 2).
CONCLUSIONS:
Similar to previous observations in pts with MF, baseline Hb levels in the control arm of the COMFORT studies were prognostic for OS. However, the introduction of RUX appears to dilute the negative prognostic effect of lower Hb on OS, while Hb decreases that occur on treatment do not adversely affect the treatment-related survival benefit. Hb decreases, likely with RUX treatment during the first 12 wks, are dose-dependent but independent of baseline spleen volume and baseline Hb. However, observations here suggest that Hb changes on RUX treatment do not bear the same prognostic implications as Hb changes that occur as a consequence of MF pathology. Together, these data suggest that transient Hb changes during treatment initiation should not lead to premature interruption or discontinuation, although appropriate supportive care and dose titration should be adapted based on a patient’s immediate needs.
Al-Ali:Celgene: Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Stalbovskaya:Novartis: Employment, Equity Ownership. Gopalakrishna:Novartis: Employment. Perez Ronco:Novartis: Employment. Foltz:Novartis: Consultancy, Honoraria, Research Funding; Incyte: Research Funding; Gilead: Research Funding; Promedior: Research Funding; Janssen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract