Abstract
Introduction: The effect of imatinib plus combination chemotherapy were assessed in 87 patients, aged 16-71 years, with newly diagnosed Philadelphia Chromosome-Positive (Ph+) acute lymphoblastic leukemia (ALL).
Methods: Imatinib (600 mg/day orally) was administered continuously with combination chemotherapy, starting from eighth day of remission induction treatment, then through 5 courses of consolidation or until allogeneic hematopoietic cell transplantation (HCT). Patients who were not transplanted were maintained on imatinib for 2 years. Molecular response monitoring was performed at the central lab (Asan Medical Center) with quantitative RT-PCR assays for peripheral blood or bone marrow BCR-ABL RNA in serial; at the time of diagnosis, at hematologic complete remission (HCR), and every 3 months thereafter. The molecular response was defined as complete (MCR) if the BCR-ABL/G6PDH ratio was less than 1x10-5.
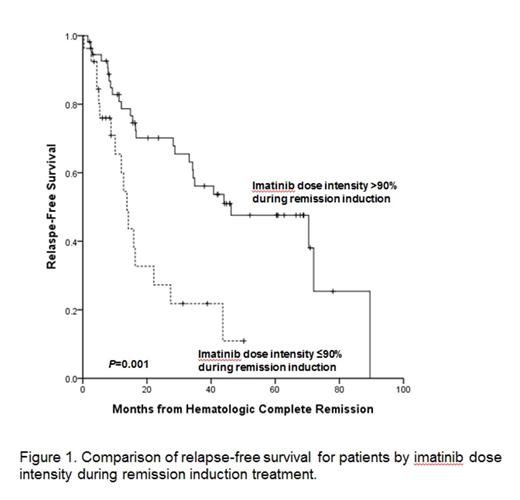
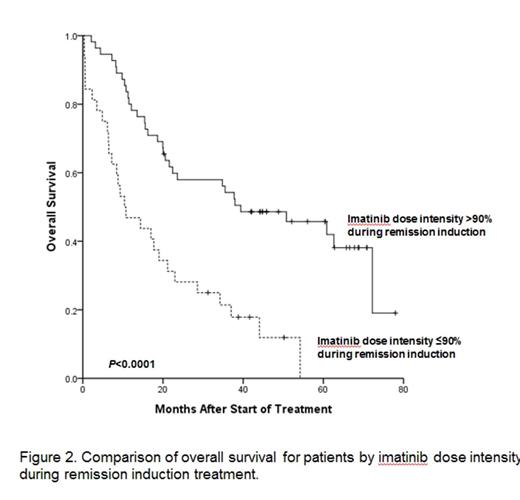
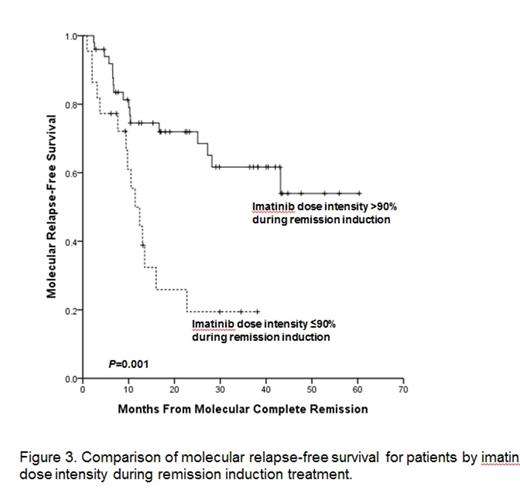
Results: Between October 2005 and February 2009, total 89 patients with newly diagnosed Ph+ALL were enrolled. With median follow-up of 5 years among survivors (range: 2.6-8.9 years) and data were frozen up in July, 2014. Two patients were not assessed, one due to a final diagnosis of CML blastic phase and one for refusal of the protocol treatment 4 months after enrollment. Eighty-two patients (94%) achieved HCR at a median 25 days (range, 14-69 days). Among these 82 HCR patients, 40 experienced recurrence of leukemia and 5-year relapse free survival (RFS) rate was 36.8%. Median time of RFS was 33 months (95% CI 20-46 months). In all, 24 patients died without leukemia progression or recurrence. Causes of treatment related morality were infection (n=5), bleeding (n=2), and HCT related complication (n=17). The 5-year overall survival (OS) rate was 33.4% and the median time of OS was 22.9 months (95% CI, 7.95-37.97 months). In total, 56 patients (68%) underwent allogeneic HCT in first HCR and had received a median 2 courses (range, 0-5 courses) of consolidation prior to HCT. At a median follow-up of 5-years (range, 2.1-8.4 years) after HCT, 23 patients experienced leukemia recurrence (cumulative incidence, 59.1%; 95% CI, 49.7%-68.5%). Of these 23 patients, 17 showed new molecular evidence of disease recurrence before hematologic relapse. Six patients, however, experienced hematologic recurrence without preceding molecular evidence of leukemia recurrence. The 5-year OS rate of patient underwent allogeneic HCT at first HCR was 52.6% and the median time of OS was 72.0 months (95% CI, 17.49-126.50 months). In the patients who completed the five cycles of consolidation, 7 patients were maintained on imatinib. Among these 7 patients, four patients finished 2-year imatinib maintenance. At median follow-up of 4 years (range, 1.9-7.4 years) after maintenance, 6 patients relapsed. The median time of RFS of patient who received maintain therapy was 40.7 months (95% CI, 24.38-57.19 months). One patient with relapse received HCT at second HCR after salvage therapy and two patients died with leukemia recurrence. Cumulative MCR rate was 88.5%, and median time to MCR was 54 days (range, 13-384 days). Median time of MCR duration was 13 months (range, 0.9-60.3 months). MCR achievement within 3months after remission induction was significant predictor of RFS (P=0.004) and OS (P=0.003). Thirty two patients who lost of MCR had significantly inferior RFS (P<0.0001) and OS (P=0.001) then 41 who maintained MCR. Total mean imatinib dose intensity over the entire treatment period was 80% (range, 22-110%) and mean imatinib dose intensity during remission induction was 85% (range, 22-131%). Imatinib dose intensity during remission induction; >90% vs. ¡Â90%; was significantly associated with median HCR duration (44 vs. 13 months, P=0.001, Fig. 1), median overall survival (39 vs. 10 months, P<0.0001, Fig. 2), and 3-year MCR rate (61% vs. 19%, P=0.001, Fig. 3). The probability for maintaining MCR at 3 years according to total imatinib dose intensity; >80% vs. ¡Â80%; was 57% (95% CI, 43.0-75.5%) and 33% (95% CI, 12.3-55.4%), respectively (P=0.05).
Conclusions: The higher imatinib dose intensity is correlated with the better molecular response and the superior overall outcome. The quantitative monitoring of BCR-ABL transcript levels is useful in identifying subgroups of Ph+ALL patients at a high risk of relapse.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.